Over the last few years, left atrial appendage occlusion (LAAO) has gained traction in patients with nonvalvular atrial fibrillation as an alternative to oral anticoagulation to prevent cerebral infarction, especially in patients with some sort of contraindication to these drugs.1
In an article published in REC: Interventional Cardiology, Ruiz-Salmerón et al.2 describe their experience using this technique during the last 10 years. This publication gives us the opportunity to review the cumulative scientific evidence available in this regard that has justified its exponential growth.
In the last national registry published in the United States, the number of physicians and hospitals that perform this intervention has gone from 30 to over 1200 and from 20 to over 400, respectively, within the last 2 years.3 In Spain, according to the registry published by the Interventional Cardiology Association of the Spanish Society of Cardiology, the number of procedures performed within the last 4 years has tripled.4
Left atrial appendage isolation started as a surgical technique back in the 1950s,5 but it was not until the beginning of 2000 when the development of percutaneous interventional procedures finally put this technique on the map.6 However, the turning point was 2009 with the publication of the multicenter and randomized PROTECT AF clinical trial7 that compared the LAAO in over 450 patients implanted with the Watchman device (Boston Scientific, United States) vs conventional treatment with warfarin. It proved the non-inferiority of the intervention for the primary composite of stroke, cardiovascular death or systemic embolism.5 Five years later, the PREVAIL trial8, with a similar design to the PROTECT AF, achieved similar results regarding efficacy, but with success rates over 95% and significantly less common complications (1.9%).
The mid-term follow-up results are even more interesting. At the 3.8-year follow-up, the patients of the PROTECT AF9 experienced a significant benefit in the composite primary endpoint (8.4% vs 13.9%; hazard ratio = 0.61; 95% confidence interval, 0.38-0.97; P = .04) compared to the control group with warfarin. Actually, even all-cause mortality improved in the LAAO group (12.3% vs 18%; hazard ratio = 0.66; 95% confidence interval, 0.45-0.98; P = .04).
Also, the third large randomized clinical trial, the PRAGUE-17,10 that compared LAAO with direct-acting oral anticoagulants in 400 patients, proved the non-inferiority of this procedure compared to new anticoagulants to prevent cardiovascular, neurological or hemorrhagic events associated with atrial fibrillation.
A meta-analysis of these randomized clinical trials proved that LAAO has similar cerebral infarction rates to those of oral anticoagulation (warfarin or new anticoagulants) with significant reductions of cerebral hemorrhages and cardiac and non-cardiac death.11
Added to this, large scale multicenter registries have proven the efficacy and safety of this intervention in patients with contraindications to oral anticoagulation. The EWOLUTION registry of 1021 patients reported a 62% rate of contraindication to oral anticoagulation, a 98.5% success rate, and a 2.7% rate of complications.12 At the 2-year follow-up, cerebral infarction rates of 1.3/100 patients-year (a 83% reduction compared to the historic series) and hemorrhage rates of 2.7/100 patients-year (a 46% reduction compared to the historic series) were reported.13 In line with this, the multicenter registry of the Amulet device (Abbott, United States) that included 1088 patients of whom 83% had contraindications to oral anticoagulation revealed a 99% success rate and a 3.2% rate of complications.14 These results are consistent with almost all the studies published over the last decade.
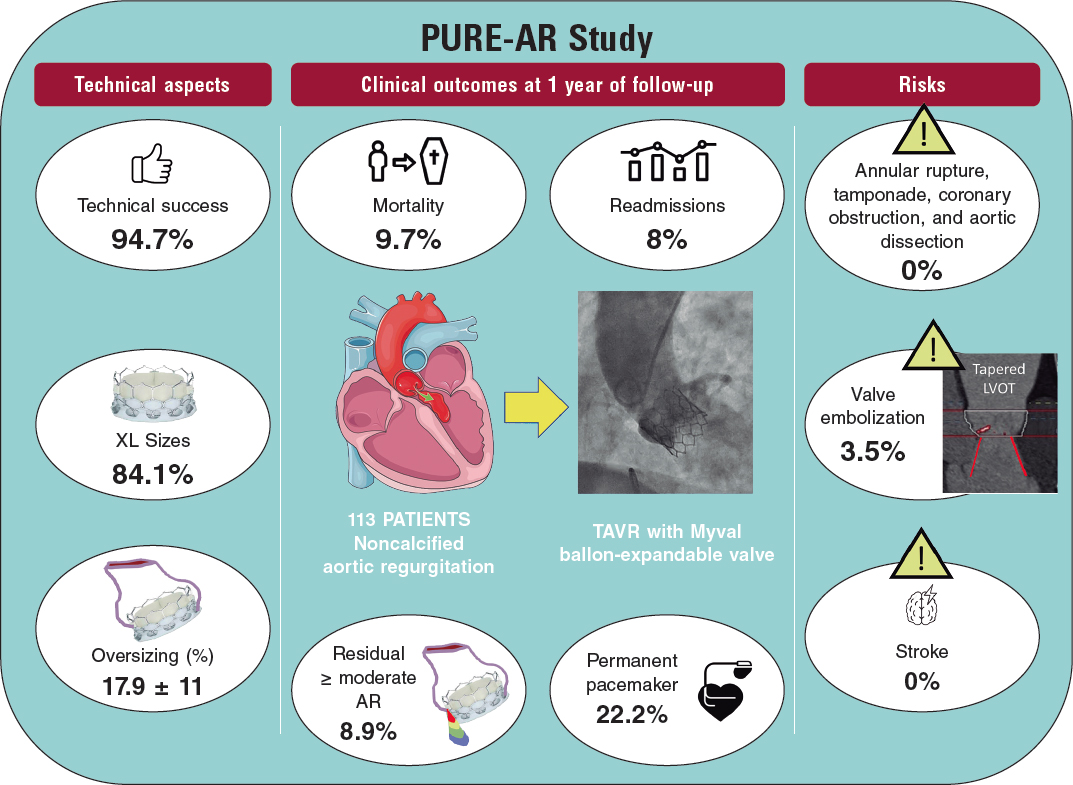
In our setting we have registries like the one published in this issue of REC: Interventional Cardiology, where Ruiz-Salmerón et al.2 analyze 260 consecutive cases of LAAO in a population of high embolic (CHA2DS2-VASc of 4.3 ± 1.6) and hemorrhagic risk (HAS-BLED of 3.7 ± 1.2). They confirmed a 75.5% reduction of embolic risk and a 58.5% reduction of hemorrhagic risk with respect to the risk predicted by both scales. Also, patients with longer follow-up periods (> 4 years in this case) showed a progressive benefit derived from the intervention (rate of events per 100 patients-year: 0.7 vs 2.0, P = .17 for embolisms; and 1.7 vs 4.0, P = .09 for major hemorrhages) compared to those with shorter follow-up periods.
Studies like this are necessary since we don’t have too many studies on long-term experiences with LAAO with mean follow-up periods > 2.5 years. It is only from this long-term follow-up perspective that we will be able to understand the impact of an intervention largely, based on the prophylaxis of the thromboembolic complications that may occur during a patient’s lifetime.
Finally, we should mention that scientific societies like the European Heart Rhythm Association and the European Association of Percutaneous Cardiovascular Interventions15 support the benefits of LAAO in situations of contraindication to oral anticoagulation, high risk of bleeding, cerebral infarction under anticoagulation or even in patients who, after being properly informed, reject oral anticoagulation. However, the current clinical practice guidelines published by the European Society of Cardiology1 still assign a low level of recommendation (IIb-B) for patients with atrial fibrillation contraindicated to long-term courses with oral anticoagulants (eg, patients with intracranial hemorrhages without reversible cause). In any case, the results of 2 ongoing large scale randomized clinical trials of LAAO vs direct-acting oral anticoagulants, the CHAMPION-AF (NCT04394546) and the CATALYST (NCT04226547) will conclusively establish the level of recommendation of this technique in patients without contraindications to oral anticoagulation.
In conclusion, we should assert that the LAAO is an effective and safe technique. With the cumulative data obtained over the last decade, its utility is undeniable in patients with atrial fibrillation who cannot take oral anticoagulation to prevent the occurrence of strokes. Also, clinical trials have proven its advantages vs warfarin, and even the long-term follow-up of these patients has offered significant positive results, even reducing mortality rate compared to oral anticoagulation. The results of the new clinical trials vs direct-acting oral anticoagulants will determine the large-scale future of this procedure.
FUNDING
I. Cruz-González has received research grants from the Instituto de Salud Carlos III (PI19/00658) and the regional health management of the Board of Castille and León (GRS 3031/A/19).
CONFLICTS OF INTEREST
I. Cruz-González is a proctor for Boston Scientific, Lifetech and Abbott, and a consultant for IHT and Qatnamedical. D. González-Calle declared no conflicts of interest whatsoever.
REFERENCES
1. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373-498.
2. Ruiz-Salmerón RJ, Ronquillo-Japón M, Carlos Robles-Pérez, et al. Una década de cierre percutáneo de la orejuela izquierda:desde el procedimiento hasta el beneficio a largo plazo. REC Interv Cardiol. 2021;3:112-118.
3. Freeman JV, Varosy P, Price MJ, et al. The NCDR Left Atrial Appendage Occlusion Registry. J Am Coll Cardiol. 2020;75:1503-1518.
4. Ojeda S, Romaguera R, Cruz-González I, Moreno R. Spanish Cardiac Catheterization and Coronary Intervention Registry. 29th Official Report of the Interventional Cardiology Association of the Spanish Society of Cardiology (1990-2019). Rev Esp Cardiol. 2020;12:927-936.
5. Madden JL. Resection of the left auricular appendix;a prophylaxis for recurrent. J Am Med Assoc. 1949;140:769-772.
6. Sievert H, Lesh MD, Trepels T, et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation:Early clinical experience. Circulation. 2002;105:1887-1889.
7. Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation:a randomised non-inferiority trial. Lancet. 2009;374:534-542.
8. Holmes DR, Kar S, Price MJ, et al. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy:The PREVAIL trial. J Am Coll Cardiol. 2014;64:1-12.
9. Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation:a randomized clinical trial. JAMA. 2014;312:1988-1998.
10. Osmancik P, Herman D, Neuzil P, et al. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients With Atrial Fibrillation. J Am Coll Cardiol. 2020;75:3122-3135.
11. Turagam MK, Osmancik P, Neuzil P, Dukkipati SR, Reddy VY. Left Atrial Appendage Closure Versus Oral Anticoagulants in Atrial Fibrillation. J Am Coll Cardiol. 2020;76:2795-2797.
12. Boersma LVA, Schmidt B, Betts TR, et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device:peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37:2465-2474.
13. Boersma LV, Ince H, Kische S, et al. Evaluating Real-World Clinical Outcomes in Atrial Fibrillation Patients Receiving the WATCHMAN Left Atrial Appendage Closure Technology:Final 2-Year Outcome Data of the EWOLUTION Trial Focusing on History of Stroke and Hemorrhage. Circ Arrhythmia Electrophysiol. 2019;12:e006841.
14. Landmesser U, Schmidt B, Nielsen-Kudsk JE, et al. Left atrial appendage occlusion with the AMPLATZER Amulet device:Periprocedural and early clinical/echocardiographic data from a global prospective observational study. EuroIntervention. 2017;13:867-876.
15. Glikson M, Wolff R, Hindricks G, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion —an update. EuroIntervention. 2020;15:1133-1180.
* Corresponding author: Servicio de Cardiología, Hospital Universitario de Salamanca, P.º San Vicente 58-182, 37007 Salamanca, Spain.
E-mail address: cruzgonzalez.ignacio@gmail.com (I. Cruz-González).