WHAT DO WE KNOW ABOUT FRACTIONAL FLOW RESERVE AFTER STENTING?
The introduction of the concept of fractional flow reserve (FFR) in the mid 90s moved coronary physiology from experimental science to routine use at the cath lab.1-3 Added to the better understanding of basic physiological mechanisms such as self-regulation and compensatory vasodilation and the coronary flow reserve introduced 20 years earlier by Gould et al.,4 FFR has undeniably changed our interpretation of coronary angiograms and had a major influence on the clinical decision-making process, and patient outcomes.3,5,6 This has resulted in the unique adoption of FFR as the only physiological index with a class I A indication for use in the clinical practice guidelines produced by the most important cardiologic societies worldwide.7,8
FFR has taught us that coronary angiography and the anatomic images we can acquire at the cath lab only provide moderately reliable measures of the functional significance of coronary artery disease and myocardial ischemia. Also, there is undeniable proof that the decision-making process based on functional measurements leads to better outcomes compared to angiography alone.3,5,6
In contrast, the interpretation of FFR after coronary intervention is still ambiguous. Basically, we should be aware that the status of a coronary artery immediately after a percutaneous coronary intervention (PCI) changes and is prone to much more variability compared to a situation of chronic stable coronary artery disease. An excellent result of a PCI performed today can change rapidly within hours due to thrombus formation, progressive dissection or other unforeseen complications. Therefore, the FFR measured immediately after a PCI should be interpreted with caution. Also, whereas the ischemic FFR threshold (0.80) in the stable angina has been clearly established, FFR values within the first days, weeks or months after a PCI can change quickly due to the healing process in the coronary artery itself, intimal hyperplasia, thrombus formation, etc. This means that by definition, the FFR after a PCI is more dynamic and that an adequate FFR value immediately after a PCI should be considerably higher than 0.80 to compensate for any intravascular changes that may occur in the short-term.
Therefore, it is not surprising to see FFR values of at least 0.90 in medical literature as indicative of an acceptable PCI result.9,10 Having said that, at the same time we should realize that most post-stent FFR values in earlier studies were obtained in patients with focal stenosis and without much diffuse disease. It is plausible to think that if diffuse disease is present inside a coronary artery, FFR values ≥ 0.90 will probably not be achieved by just stenting a focal stenosis.
Most likely, in such cases, hyperemic pressure pullback recording (whether motorized or not) or a sophisticated variety called hyperemic pressure pullback gradient will better qualify the functional result after stenting. They will also reveal if the remaining gradients inside the coronary artery are due to insufficient stent deployment or more diffuse disease.11
Despite these limitations, the existence of a clear correlation between the FFR values measured immediately after PCI and long-term outcomes is undeniable. This was first described in the FFR-post-STENT Registry of 750 patients that revealed the existence of an inverse correlation between the FFR values measured immediately after the PCI and the rate of restenosis at the 6-month follow-up (figure 1). Such an inverse correlation between high FFR values post-stenting and the mid-term risk of restenosis has been confirmed ever since.12 Still, it is not completely clear if suboptimal FFR values after stenting are due to a focal problem in the stented segment or to diffuse disease elsewhere in the artery. Hyperemic pressure pullback recording and pressure pullback gradient recording have proven that a considerable pressure gradient across the stent is often associated with inappropriate deployments as seen on intravascular ultrasounds or optical coherence tomographies.
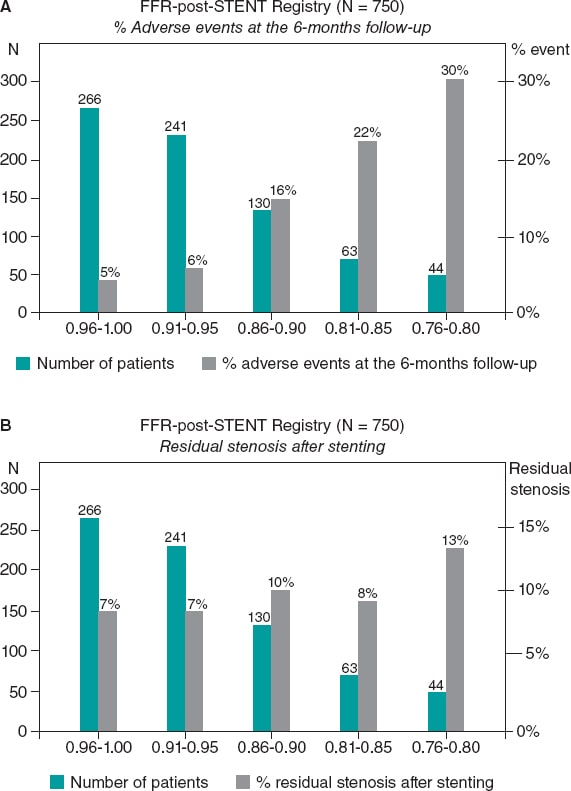
Figure 1. Superiority of functional testing compared to angiography alone to predict the outcomes after stenting. A: correlation between post-stenting fractional flow reserve (FFR) and the rate of adverse events at the 6-month follow-up in 750 patients from the FFR-Post-Stent registry. B: correlation between post-stenting angiography and the rate of adverse events at the 6-month follow-up. Reproduced with permission from Pijls et al.9
PATIENT AND VESSEL RELATED PREDICTORS OF POST-PROCEDURAL FFR
At this point, the study conducted by van Zandvoort et al. and recently published on REC: Interventional Cardiology comes into perspective.10 In this study, a large registry of 1000 consecutive patients from 1 large volume cardiac center, the FFR was consistently measured after an angiographically successful PCI without the intention of performing any additional procedures in cases of unexpectedly low FFR values. The authors should be praised for their adherence to this protocol. Afterwards, independent patient and vessel related characteristics were determined as associated with the post-procedural FFR values. Considering that in this study no information was available on the outcomes and that the relationships were not causal per se but associative, a few important lessons can be learned.
In the first place, a very strong predictor of lower FFR values was stent implantation in the left anterior descending coronary artery (LAD). In truly normal LADs, the FFR is not different from other arteries and is very close to 1.00.13 However, even in cases of mild disease, the FFR values measured in the LAD are often more damaged compared to other arteries. This is explained by the fact that the LAD perfusion territory is large. One of the major advantages of FFR with respect to other methodologies that only address a coronary artery injury is that the FFR does not only measure the stenosis itself, but also the extent of the perfusion territory. If a similar stenosis (with similar angiographic, intravascular ultrasound or optical coherence tomography characteristics) is located in a coronary artery with a larger perfusion territory, the FFR will be lower. In this regard, the FFR is actually the link between stenosis severity, coronary blood flow, extent of the myocardial perfusion territory, and myocardial ischemia.14 As such, it is plausible that after a successful PCI, the FFR of the LAD will be somehow lower compared to other coronary arteries.
A second interesting observation is that in women, the FFR measured after apparently successful stenting was often higher compared to the males. Van Zandvoort et al. suggest that this might be due to the fact that in women, microvascular disease plays a more predominant role compared to men. Also, that the generation of a hyperemic gradient within the epicardial coronary artery may be blunted by the presence of microvascular disease.10 To confirm that position, more detailed studies of coronary microvasculature are needed. For many years, the assessment of microvascular disease in a true quantitative way has been too hard to pin down. However, recently developed methodology has changed that as follows.15
SIMULTANEOUS ASSESSMENT OF EPICARDIAL AND MICROVASCULAR DISEASE
Recently, the technique for measuring absolute coronary blood flow and microvascular resistance has been introduced as an adjunctive to FFR measurement. This technique is based on the continuous infusion of a saline solution at a low rate and thermodilution. The technique is simple, elegant, accurate, and operator independent.15
In short, immediately after measuring the FFR, a specifically designed infusion catheter (Rayflow, Hexacath, Paris) is advanced while mounted on the pressure guidewire and placed inside the stent (to study the microvascular resistance of the territory distal to the stent). Then, the infusion of a saline solution at a low rate is started and the absolute coronary blood flow in mL/min is measured in the area of interest using this equation:
Q = Qi × T/Ti × 1.08
where Q is blood flow in the myocardial territory distal to the stent, Qi is the infusion rate of the saline solution (mL/min), Ti is the temperature of the infused saline solution (°C), and T is the temperature in the distal coronary artery after mixing blood and the saline solution. T and Ti are expressed as the difference with respect to body temperature.
With infusion rates = 8 to 10 mL/min, resting blood flow values are obtained and with infusion rates = 20 mL/min, maximum hyperemic values are obtained since the saline solution itself at that rate induces maximum hyperemia in a matter of seconds (figure 2; unpublished data).
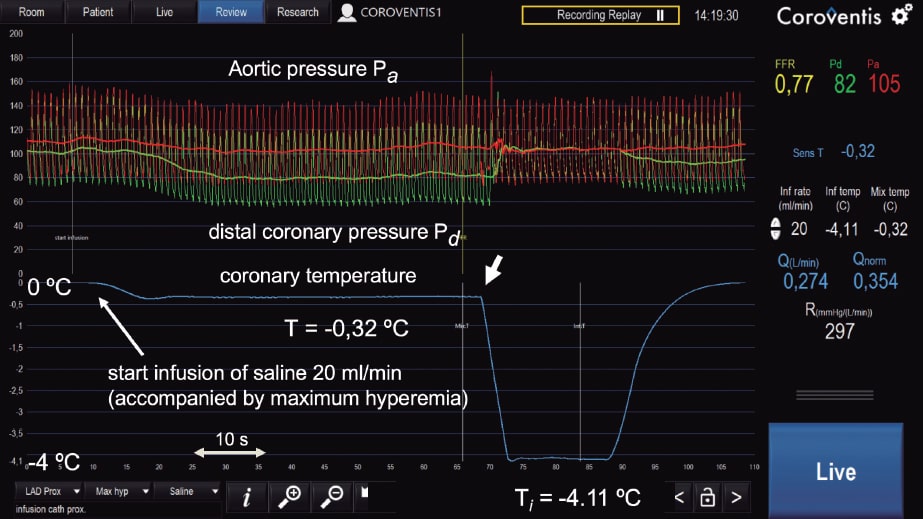
Figure 2. Example of absolute coronary blood flow and microvascular resistance measurement in left anterior descending coronary artery following routine fractional flow reserve measurement. On the figure upper side, aortic pressure (Pa), and distal coronary pressure (Pd) are shown in red and green, respectively. The blue line represents coronary temperature as a difference with respect to body temperature (T = 0). After starting the infusion of 20 mL/min of a saline solution (Qi) at room temperature through a dedicated infusion catheter, maximum hyperemia occurs within seconds. Then, after a complete mix of blood and saline solution, a steady-state distal coronary temperature T of 0.32 oC below body temperature is achieved. After withdrawing the pressure/temperature sensor from the tip of the infusion catheter, the infusion temperature (Ti) measured is 4.11 oC below body temperature. Absolute flow (Q) in the coronary artery is calculated using the equation shown in this article (274 mL/min). Microvascular resistance is simply calculated now by Pd/Q and equals 297 Wood units, which is a normal value for the anterior wall. Since the fractional flow reserve value is also known, the normal reference value of coronary flow can be calculated. All parameters are shown online supported by dedicated software (Coroventis, Sweden).
Immediately after the simultaneous measurement of distal coronary pressure and blood flow values, quantitative microvascular resistance Rµ (Wood units) is estimated. In this same way, epicardial disease (indicated by FFR) and microvascular disease (indicated by Rµ) can be assessed and independently distinguished. The position taken by van Zandvoort et al. suggesting that higher FFR values after PCI are related to a higher microvascular resistance in women could be elegantly validated this way.
WHAT IS THE BEST TECHNIQUE TO MEASURE THE FFR VALUES AFTER A PCI?
In the study conducted by van Zandvoort et al. the FFR values measured after the PCI are only briefly described.10 The authors do not report if hyperemic pressure pullback recordings were performed or other techniques used to assess the entire artery. Also, we should mention that to measure intracoronary pressure, not a single true pressure guidewire was used, but the Navvus system instead (ACIST Medical Systems, Eden Prairie, MN, United States). This system is known to overestimate pressure gradients and underestimate FFR mildly in cases of minimal disease or moderately in cases of more severe disease.16 Also, this system is not validated against regular pressure guidewires in post-PCI vessels.
Nevertheless, the measurements were taken meticulously using IV adenosine at a rate of 140 µg/kg/min, giving the opportunity of an easy and reliable estimation of the FFR under stable conditions. We should mention that only 2 out of 1000 patients showed adenosine intolerance, meaning that the infusion of adenosine had to be interrupted due to harmless adenosine-induced, angina-like chest pain. This underlines the safety profile of IV adenosine infusions as seen in tens of thousands of patients in a myriad of other studies.
In future studies on the meaning and interpretation of post-PCI FFR, it would be adviseable to perform hyperemic pressure pullback recordings as outlined in the first part of this article or use the even more sophisticated technique for whole vessel evaluation after PCI recently introduced by Collet et al. and called hyperemic pressure pullback gradient.11 Obviously, all pressure analyses performed inside the stented coronary artery should preferably be performed at maximum hyperemia since gradients at rest inside the artery are 2 to 3 times smaller. Consequently, the signal-to-noise ratio of a resting pullback recording is 2 to 3 times less sensitive.
In conclusion, although outcome data were not presented which, by the way, was not the objective of the study, the interesting trial conducted by van Zandvoort et al.10 teches us about several interesting patient and vessel related predictors of post-procedural FFR measurement. Also, it anticipates the need for future physiological studies to unravel the different factors involved using new research methods on coronary circulation like hyperemic pressure pullback gradients and truly quantitative microvascular resistance (Rµ) measurements.
FUNDING
No funding whatsoever with regards to this article.
CONFLICTS OF INTEREST
N.H.J. Pijls has received institutional research grants from Abbott and Hexacath. Also, he is a consultant for Abbott, Opsens, and General Electric, and a minor stockholder of Philips, GE, ASML, and Heartflow. L.X. van Nunen declared no conflicts of interest whatsoever.
REFERENCES
1. Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87:1354-1367.
2. Pijls NH, De Bruyne B, Peels K, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703-1708.
3. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213-224.
4. Gould KL, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol. 1974;33:87-94.
5. De Bruyne B, Fearon WF, Pijls NH, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208-1217.
6. Zimmermann FM, Omerovic E, Fournier S, et al. Fractional flow reserve-guided percutaneous coronary intervention vs. medical therapy for patients with stable coronary lesions:meta-analysis of individual patient data. Eur Heart J. 2019;40:180-186.
7. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477.
8. Patel MR, Calhoon JH, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease:A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for T. J Am Coll Cardiol. 2017;69:2212-2241.
9. Pijls NH, Klauss V, Siebert U, et al. Coronary Pressure Measurement After Stenting Predicts Adverse Events at Follow-Up. Circulation. 2002;105:2950-2954.
10. van Zandvoort LJC, Masdjedi K, Neleman T, et al. Predictors of postprocedural fractional flow reserve:insights from the FFR-SEARCH study. REC Interv Cardiol. 2020. 2021;3:91-97.
11. Collet C, Sonck J, Vandeloo B, et al. Measurement of Hyperemic Pullback Pressure Gradients to Characterize Patterns of Coronary Atherosclerosis. J Am Coll Cardiol. 2019;74:1772-1784.
12. Rimac G, Fearon WF, De Bruyne B, et al. Clinical value of post-percutaneous coronary intervention fractional flow reserve value:A systematic review and meta-analysis. Am Heart J. 2017;183:1-9.
13. Pijls NH, Van Gelder B, Van der Voort P, et al. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995;92:3183-3193.
14. De Bruyne B, Hersbach F, Pijls NH, et al. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but 'Normal'coronary angiography. Circulation. 2001;104:2401-2406.
15. Xaplanteris P, Fournier S, Keulards DC, et al. Catheter-Based Measurements of Absolute Coronary Blood Flow and Microvascular Resistance:Feasibility, Safety, and Reproducibility in Humans. Circ Cardiovasc Interv. 2018;11:e006194.
16. Wijntjens GW, van de Hoef TP, Kraak RP, et al. The IMPACT Study (Influence of Sensor-Equipped Microcatheters on Coronary Hemodynamics and the Accuracy of Physiological Indices of Functional Stenosis Severity). Circ Cardiovasc Interv. 2016;9:e004645.
* Corresponding author: Department of Cardiology, Catharina Hospital, P.O. Box 1350, 5602 ZA Eindhoven, The Netherlands.
E-mail address: lokien.v.nunen@catharinaziekenhuis.nl (L.X. van Nunen).











