To the Editor,
The use of circulatory support has grown exponentially over the last decade, particularly for the management of cardiogenic shock in the setting of acute myocardial infarction.1,2 The devices more often used like the Impella CP (Abiomed, United States) show good results in observational studies. These studies describe an improved survival rate when these devices are used as part of a well-defined program to treat cardiogenic shock.3-5 However, this is not a risk-free therapy, and device displacement is a complication that can occur while the patient is being moved or transferred. Although rare, this complication can be deadly if not solved immediately because there is a loss of hemodynamic support. In these cases, the device needs to be retrieved due to the impossibility of crossing the aortic valve to proceed with a new implant.
We present a new technique for the emergent percutaneous repositioning of the Impella CP device as performed in 3 cases in 2 different hospitals. Informed consent was obtained from the patients or their relatives for the publication of their cases.
The complete displacement of the Impella CP device towards the aorta poses several technical difficulties regarding repositioning. In the first place, it is not easy to cross the aortic valve with the device just by pushing it; what will probably happen is that it will crash into 1 of the leaflets running the risk of damaging them. Secondly, it cannot be mounted over a conventional 0.035 in guidewire, only over a special 0.018 in guidewire, that happens to be unavailable in the cath lab. Also, it needs to be inserted through a guidewire that runs across the Impella CP device motor and can be retrieved after implantation, which is why a correct reinsertion is very difficult to achieve, if not impossible. Lastly, if the 14-Fr introducer sheath has been removed, as it is usually the case to extract and reinsert the Impella CP device, the artery needs to be recanalized with a new introducer sheath. For all these reasons, the way to reverse the Impella CP displacement is usually to replace it completely with the corresponding delay and high cost.
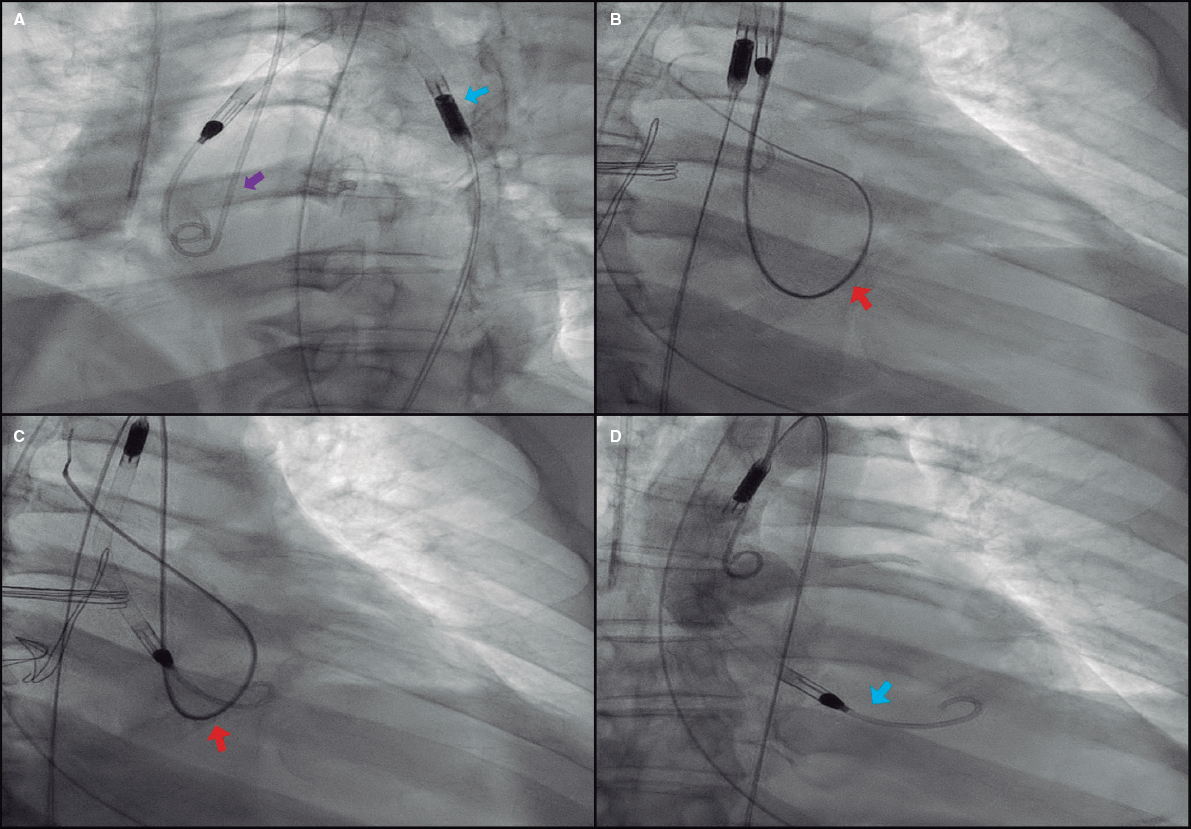
The reinsertion technique presented here consists of facilitating the aortic valve crossing through an easy maneuver that keeps it open for a few seconds. Using the radial access and a 0.035 in guidewire a 5-Fr/6-Fr pigtail catheter is advanced towards the left ventricle and pushed until it spins around the ventricle to eventually exit through the aortic valve. The guidewire should be kept inside the catheter for further support. This in-and-out loop keeps the valve open, which allows an easy advance of the Impella CP device until its correct positioning (figure 1); this last maneuver should be performed carefully to avoid vascular complications. If it encounters any sort of resistance in the valvular plane, it is pulled back just a few centimeters and a new attempt is made with a small rotation. In 1 of the cases reported the valve could not be crossed during the first attempt and in the other 2 cases, 3 or 4 attempts were needed. However, the valve was always crossed in a few minutes and without immediate complications (video 1 of the supplementary data). Although significant aortic regurgitation almost surely occurs while performing the maneuver, it was well tolerated in all cases, probably due to its short duration and added benefit of restoring circulatory support.
Figure 1. Impella CP device repositioning technique. A: pigtail catheter insertion (purple arrow) via radial access. Note how the Impella CP catheter has been displaced towards the ascending aorta (blue arrow). B: formation of intraventricular loop with the pigtail catheter keeping the 0.035 in guidewire to improve circulatory support (red arrow) while running across the aortic annulus, which facilitates its exit towards the aorta and keeps the valve open. C: afterwards, the Impella CP catheter (red arrow) is carefully advanced and pulled back by performing small rotations, if necessary, until reaching its final position (D, blue arrow).
From December 2015 through December 2019, 97 Impella CP devices were implanted in our hospitals. In 7 of them (7.2%) catheter displacement was reported. In 3 of the cases, the displacement was limited to the outflow tract and repositioned at the intensive care unit. In the remaining 4 cases, the displacement was total, the catheter was retrieved from the left ventricle all the way to the aorta and hemodynamic support was lost. In 1 of these patients, support was being withdrawn, which is why the device was eventually removed. The remaining 3 required the emergent repositioning of the device using the technique described here. Table 1 shows the characteristics of these patients. The procedure was successful and without complications, and circulatory support was immediately recovered in the 3 cases. However, due to these patients’ critical condition, 2 of them died a few days later of irreversible multiple organ failure.
Table 1. Characteristics of patients in whom the Impella CP device was repositioned
| Case #1 | Case #2 | Case #3 | |
|---|---|---|---|
| Age (years) | 66 | 75 | 46 |
| Sex | Male | Male | Male |
| Reason for the implant | Cardiogenic shock in the AMI setting | Electrical storm | LV unloading (APE after ECMO) |
| Previous LVEF (%) | 20 | 20 | 5-10 |
| Previous lactate (mmol/L) | 5.1 | 4.2 | > 15 |
| Vasoactive drugs | NA + DBT | NA | NA + DBT |
| Additional support | ECMO | ECMO | ECMO |
| Impella access | Left femoral access | Right femoral access | Right femoral access |
| Cause of displacement | Transfer of the patient | Mobilization during x-ray | Accidental removal at the operating room |
| Successful repositioning | Yes | Yes | Yes |
| Hemodynamic improvement | Yes | Yes | Yes |
| Survival | No | No | Yes (transplant) |
AMI, acute myocardial infarction; APE, acute pulmonary edema; DBT, dobutamine; ECMO, extracorporeal membrane oxygenation; LV, left ventricle; LVEF, left ventricular ejection fraction; NA, noradrenaline. | |||
In conclusion, we presented a safe, efficient, cost-effective, and rapid technique that could be widely used to solve the Impella CP device displacement, minimize its potential consequences, and reduce costs.
FUNDING
No funding has been received.
AUTHORS' CONTRIBUTION
M.E. Vázquez, T. Bastante, and E. Gutiérrez-Ibañes conducted the procedures. J. García-Carreño and E. Gutiérrez-Ibañes wrote the article. M.E. Vázquez, T. Bastante, F. Fernández-Avilés and F. Alfonso supervised and corrected the article.
CONFLICTS OF INTEREST
F. Alfonso is associate editor of Rev Esp Cardiol. The journal’s editorial procedure to ensure impartial handling of the manuscript has been followed.
SUPPLEMENTARY DATA
Video 1. García-Carreño J. DOI: 10.24875/RECICE.M20000156
REFERENCES
1. Berg D, Barnett C, Kenigsberg B, et al. Clinical Practice Patterns in Temporary Mechanical Circulatory Support for Shock in the Critical Care Cardiology Trials Network (CCCTN) Registry. Circ Heart Fail. 2019;12:e006635.
2. Amin AP, Spertus JA, Curtis JP, et al. The Evolving Landscape of Impella®Use in the United States Among Patients Undergoing Percutaneous Coronary Intervention with Mechanical Circulatory Support. Circ. 2020;141:273-284.
3. Basir MB, Kapur NK, Patel K, et al. Improved Outcomes Associated with the use of Shock Protocols:Updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2019;93:1173-1183.
4. Basir MB, Schreiber T, Dixon S, et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock:The Detroit cardiogenic shock initiative. Catheter Cardiovasc Interv. 2018;91:454-461.
5. Tehrani B, Truesdell A, Singh R, Murphy C, Saulino P. Implementation of a Cardiogenic Shock Team and Clinical Outcomes (INOVA-SHOCK Registry):Observational and Retrospective Study. JMIR Res Protoc. 2018;7:e160.
Corresponding author: Servicio de Cardiología, Hospital General Universitario Gregorio Marañón, Dr. Esquerdo 46, 28007 Madrid, Spain.
E-mail address: egutibanes@gmail.com (E. Gutiérrez-Ibañes).