ABSTRACT
Introduction and objectives: patients with long, sequential and diffuse coronary lesions who undergo a percutaneous coronary intervention remain at a high risk of suffering cardiovascular events despite the improved safety and efficacy of the new drug-eluting stents. The objective of this study was to analyze the utility of SyncVision/iFR-guided revascularization (SyncVision version 4.1.0.5, Philips Volcano, Belgium) in this type of lesions.
Methods: Randomized, multicenter, controlled, and open-label trial designed to compare SyncVision/iFR-guided and angiography-guided revascularizations in patients with long, sequential or diffuse significant angiographic coronary stenosis (ClinicalTrials.gov identifier: NCT04283734). A total of 100 patients will be randomized (1:1, no stratification). The primary endpoint is the average length of the stent implanted. The secondary endpoint is a composite of cardiac death, myocardial infarction, definitive or probable stent thrombosis, new target lesion revascularization or new target lesion failure; and the presence of residual ischemia as seen on single-photon emission computed tomography at the 6-month follow-up. Patients will be followed for 12 months after the procedure.
Results: The trial is currently in the recruitment phase, and it has already recruited the first 7 patients. We expect to complete the recruitment phase by February 2021 and the follow-up by February 2022.
Conclusions: The iLARDI study is the first randomized trial to assess the potential utility of SyncVision-guided revascularization in long, sequential and diffuse coronary lesions.
Keywords: Diffuse coronary artery disease. Long coronary artery disease. Instantaneous wave-free ratio. SyncVision software.
RESUMEN
Introducción y objetivos: Los pacientes con lesiones coronarias largas, secuenciales o difusas tratadas percutáneamente continúan presentando un riesgo alto de eventos cardiovasculares a pesar de la mejoría de la seguridad y de la eficacia de los nuevos stents liberadores de fármacos. El objetivo de este estudio es analizar la utilidad del software SyncVision/iFR (SyncVision versión 4.1.0.5, Philips Volcano, Bélgica) para guiar la revascularización en este tipo de lesiones.
Métodos: Estudio aleatorizado, multicéntrico, controlado y abierto para comparar la revascularización guiada por SyncVision/iFR respecto a la revascularización guiada por angiografía en pacientes con lesiones coronarias largas, secuenciales o difusas (identificador de ClinicalTrials.gov: NCT04283734). Se incluirá a 100 pacientes (aleatorización 1:1 no estratificada). El objetivo primario es la longitud total del stent implantado. Como objetivo secundario se ha establecido un combinado de muerte cardiaca, infarto de miocardio, trombosis definitiva o probable del stent, nueva revascularización de la lesión tratada en el procedimiento basal o nueva revascularización de la lesión analizada en el procedimiento basal, y la presencia de isquemia residual evaluada por tomografía computarizada por emisión de fotón simple a los 6 meses de seguimiento. El tiempo de seguimiento será de 12 meses tras el procedimiento índice.
Resultados: El estudio se encuentra actualmente en fase de reclutamiento, con los primeros 7 pacientes ya incluidos. Esperamos completar el reclutamiento en febrero de 2021 y el seguimiento en febrero de 2022.
Conclusiones: El estudio iLARDI es el primer estudio aleatorizado para la evaluación de la potencial utilidad de la revascularización guiada por SyncVision en lesiones coronarias largas, secuenciales y difusas.
Palabras clave: Lesiones coronarias difusas. Lesiones coronarias largas. Relación en el periodo instantáneo libre de ondas. Software SyncVision.
Abbreviations: PCI: percutaneous coronary intervention. iFR: instantaneous wave-free ratio. MACE: major adverse cardiovascular events.
INTRODUCTION
The physiological assessment of coronary lesions is a routine practice in contemporary cath labs and is strongly recommended by the European guidelines to guide the percutaneous coronary intervention (PCI) decision-making process.1 Unlike fractional flow reserve, the new instantaneous wave-free ratio (iFR) index allows us to analyze the physiological significance of each lesion and each coronary segment.2-5 This has led to the creation of the new and specific SyncVision software package (SyncVision version 4.1.0.5, Philips Volcano, Belgium), that shows the functional compromise of each lesion and predicts the expected iFR improvement after percutaneous treatment.3,4
Few observational studies published have analyzed the reduction in the length of the stent implanted compared to angiography-guided revascularization in long and diffuse coronary lesions.4,5 However, this reduction could be detrimental to the complete coverage of the plaque in this type of lesions, which has proven to be a predictor of major adverse cardiovascular events at the follow-up.6
The objective of our study is to analyze the utility of the iFR and SyncVision software to guide the PCI decision-making process in long, sequential, and diffuse coronary lesions.
METHODS
We have designed a multicenter, randomized, controlled, and open-label trial to compare SyncVision/iFR-guided revascularization to angiography-guided revascularization in patients with long, sequential or diffuse significant angiographic coronary lesions (ClinicalTrials.gov identifier: NCT04283734). All the variables that will be analyzed during the study are shown on table 1.
Table 1. Variables that will be analyzed during the study
| Nº | Variable | Expressed as |
|---|---|---|
| Personal medical history | ||
| 1 | Sex (men/women) | no. (%) |
| 2 | Age (years) | no. ± SD |
| 3 | Hypertension | no. (%) |
| 4 | Diabetes mellitus | no. (%) |
| 5 | Dyslipidemia | no. (%) |
| 6 | Former smoker | no. (%) |
| 7 | Previous ischemic cardiomyopathy | no. (%) |
| 8 | Previous revascularization | no. (%) |
| 9 | Atrial fibrillation | no. (%) |
| 10 | Heart failure | no. (%) |
| 11 | Previous stroke | no. (%) |
| 12 | Peripheral artery disease | no. (%) |
| 13 | Previous significant bleeding | no. (%) |
| 14 | Basal hemoglobin levels (mg/dL) | no. ± SD |
| 15 | Basal creatinine levels (mg/dL) | no. ± SD |
| 16 | Left ventricular ejection fraction (%) | no. ± SD |
| 17 | Clinical presentation (stable angina/NSTEMI/STEMI) | no. (%) |
| 18 | Baseline ultra-sensitive troponin levels (ng/L) | no. ± SD |
| Procedural data | ||
| 19 | Arterial access (radial/femoral/other) | no. (%) |
| 20 | P2Y12 inhibitor preload | no. (%) |
| 21 | IIb/IIIa inhibitor use during the procedure | no. (%) |
| 22 | Multivessel disease | no. (%) |
| 23 | Syntax score | no. ± SD |
| 24 | Randomized vessel (LAD/LCx/RCA/other) | no. (%) |
| 25 | Vessel lesion length (mm) | no. ± SD |
| 26 | Vessel reference diameter (mm) | no. ± SD |
| 27 | Vessel stenosis (%) | no. ± SD |
| 28 | Total stent length as seen on the angiography (mm) | no. ± SD |
| 29 | Total length of the stent implanted (mm) | no. ± SD |
| 30 | Differences between stent length estimated and implanted (mm) | no. ± SD |
| 31 | Stent diameter (mm) | no. ± SD |
| 32 | Optimal angiographic result (final TIMI III flow, absence of dissections and residual stenosis < 20%) | no. (%) |
| 33 | Contrast (milliliters) | no. ± SD |
| 34 | Use of intracoronary imaging | no. (%) |
| 35 | Use of rotablation | no. (%) |
| 36 | Procedural complications (no reflow/ dissection/acute vessel closure/perforation/other) | no. (%) |
| 37 | Baseline iFR in the intervention group | no. ± SD |
| 38 | Diffuse improvement of iFR by SyncVision | no. (%) |
| 39 | Estimated stent length to achieve an iFR > 0.89 (mm) | no. ± SD |
| 40 | Final iFR in the intervention group | no. ± SD |
| 41 | Need to implant an additional stent | no. (%) |
| Hospitalization data | ||
| 42 | Bleeding complications | no. (%) |
| 43 | Ultra-sensitive troponin peak levels (ng/L) | no. ± SD |
| 44 | Periprocedural myocardial infarction | no. (%) |
| 45 | In-hospital death | no. (%) |
| 46 | In-hospital stroke | no. (%) |
| 47 | In-hospital stent thrombosis | no. (%) |
| Pharmacological treatment at discharge | ||
| 48 | Aspirin | no. (%) |
| 49 | P2Y12 Inhibitor (no/clopidogrel/ticagrelor/prasugrel) | no. (%) |
| 50 | Anticoagulation (no/acenocumarol/rivaroxaban/ dabigatran/apixaban/edoxaban) | no. (%) |
| 51 | Beta-blockers | no. (%) |
| 52 | ACEI/ARB/ARNI | no. (%) |
| 53 | Calcium antagonists | no. (%) |
| 54 | Other anti-ischemic drugs | no. (%) |
| Follow-up visits (after 3, 6, and 12 months) | ||
| 55 | Bleeding complications | no. (%) |
| 56 | Dual antiplatelet therapy | no. (%) |
| 57 | Anticoagulation (no/acenocumarol/rivaroxaban/ dabigatran/apixaban/edoxaban) | no. (%) |
| 58 | Probable or definitive stent thrombosis | no. (%) |
| 59 | Spontaneous myocardial infarction | no. (%) |
| 60 | New target lesion revascularization | no. (%) |
| 61 | New target vessel revascularization | no. (%) |
| 62 | Revascularization of other vessel | no. (%) |
| 63 | Death | no. (%) |
| 64 | Cause of death (cardiac/non cardiac) | no. (%) |
| 65 | Stroke | no. (%) |
| 66 | Beta-blockers | no. (%) |
| 67 | ACEI/ARB/ARNI | no. (%) |
| 68 | Calcium antagonists | no. (%) |
| 69 | Other anti-ischemic drugs | no. (%) |
| 70 | Residual angina (I/II/III/IV) | no. (%) |
| 71 | Withdrawal from the study | no. (%) |
| 72 | Lost to follow-up | no. (%) |
ACEI, angiotensin-converting-enzyme inhibitors; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor blocker and neprilysin inhibitor; LAD, left anterior descending coronary artery; LCx, left circunflex artery; RCA, right coronary artery; SD, standard deviation; TIMI, Thrombolysis in Myocardial Infarction. NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction. | ||
Additionally, the study has received the proper ethical oversight and has been approved by the Ethical Comitee of Córdoba.
Inclusion and exclusion criteria
Patients with the following criteria are being included: a) patients > 18 years old who require percutaneous coronary treatment due to ischemia (silent, stable angina or acute coronary syndrome); b) presence of a vessel with sequential lesions separated by < 10 mm from each other with a total lesion length > 25 mm and a percent diameter stenosis > 60% (as seen on the quantitative coronary angiography assessment) in, at least, 1 segment; or a coronary segment > 30 mm with diffuse disease, and a percent diameter stenosis > 60% (as seen on the quantitative coronary angiography assessment) in, at least, 1 region; c) baseline iFR ≤ 0.89 distal to a potentially randomizable lesion.
We have stablished the following exclusion criteria: a) patients with acute coronary syndrome with non-optimal results in the culprit vessel (final Thrombolysis in Myocardial Infarction (TIMI) flow grade < III, non-reflow phenomenon during treatment, residual coronary dissection, lost or compromise of a major side branch); b) patients with acute coronary syndrome and left ventricular ejection fraction < 45%; c) life expectancy < 12 months; d) patients with severe aortic stenosis; e) contraindication for dual antiplatelet therapy for at least 12 months; f) presence of significant thrombocytopenia (< 10 x 109/L); g) patients with an indication for bypass surgery according to the heart team; h) pregnancy; i) inability to understand the informed consent.
Endpoints
The study primary endpoint is the reduction of the average length of the stent implanted in the SyncVision-guided group measured in millimeters (mm) compared to the angiography-guided group. The study secondary endpoint is a composite of cardiac death, myocardial infarction, definitive or probable stent thrombosis, new target lesion revascularization or new target lesion failure (major adverse cardiovascular events [MACE]); and the assessment of residual ischemia through single-photon emission computed tomography at the 6-month follow-up.
Procedure
After the diagnostic phase, the use of intracoronary vasodilators is mandatory to exclude possible coronary spasms. Lesions will be assessed by 2 expert operators (prior to randomization) to determine the coronary segment to treat when the revascularization is angiography-guided based on current routine clinical practice. Afterwards, the iFR will be determined at baseline. If the obtained iFR is ≤ 0.89, patients will be randomized to the angiography-guided revascularization group (the control group) or to the iFR pullback-guided revascularization group using the SyncVision software (figure 1). Intracoronary imaging can be used in both groups based on the operator’s criteria to optimize the angiographic result.
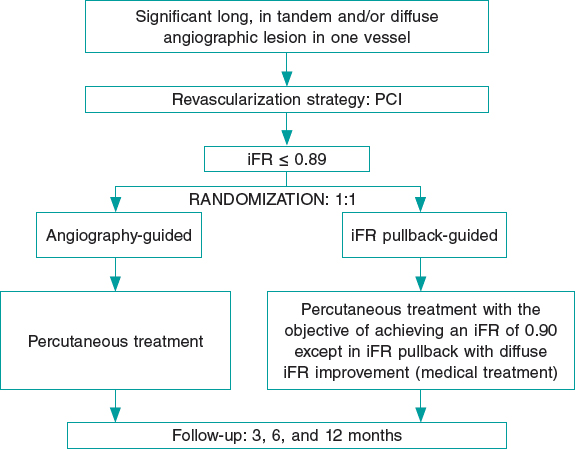
Figure 1. Summary of randomization, treatment targets, and follow-up of the iLARDI study. iFR, instantaneous wave-free ratio; MACE, major adverse cardiovascular events; PCI, percutaneous coronary intervention.
In the intervention group, a pressure wire (Verrata pressure guidewire, Philips Volcano, Belgium) will be inserted trough a guide catheter towards the vessel ostium to normalize the pressure between the aortic and the vessel ostium. Secondly, the pressure wire will be advanced distally to the lesion. Under stable hemodynamic conditions (without the administration of vasodilators), we will determine the baseline iFR. Afterwards, the wire will be removed under continuous fluoroscopy, and in the same projection. If the iFR at the vessel ostium is 1 ± 0.02, the absence of drift will be confirmed and an angiogram in the same angiographic position will be performed. The SyncVision software can recognize the vessel analyzed and identify the physiological contribution of every lesion and every segment, predicting the improvement of the iFR after treatment. The iFR improvement is depicted as yellow dots. Each yellow dot represents an iFR improvement of 0.01 if that zone was percutaneously treated. The accumulation of many yellow dots suggests that the contribution of that lesion to physiological compromise is high. After performing the physiological assessment of each lesion, the operator would have to treat the minimum segment needed to achieve an iFR of 0.90. Cases without an accumulation of dots have been considered as physiological diffuse disease (defined as the presence of < 20% of the total number of dots) in the coronary segment physiologically assessed. Those cases will be medically treated due to the theoretical absence of benefit of the percutaneous treatment (figure 2 and figure 3).
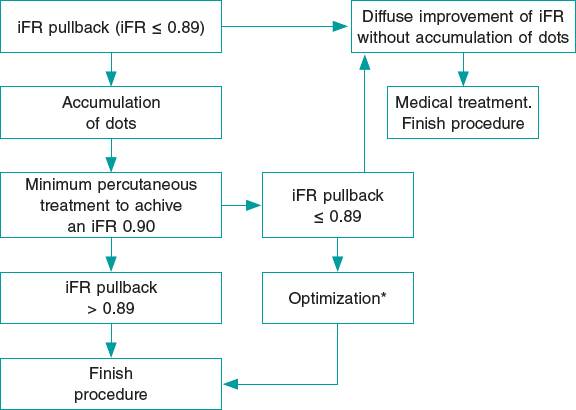
Figure 2. Flowchart of technical treatment details of patients randomized to the intervention group.
* We consider as optimization the postdilatation of the previous stented area if an in-stent accumulation of yellow dots is seen; or the percutaneous treatment of a new segment with physiological compromise not seen in the baseline iFR-pullback study. iFR, instantaneous wave-free ratio.
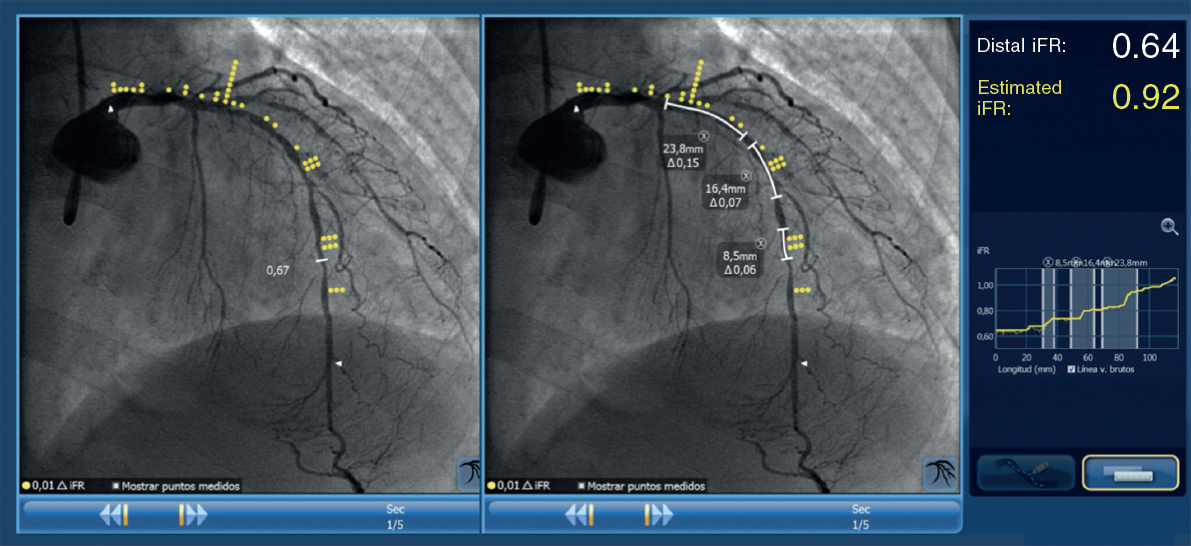
Figure 3. Image of iFR co-registration using the SyncVision software in a patient included in the study and randomized to the intervention group with a diffuse lesion in the left anterior descending coronary artery, and the physiological contribution of every segment. The estimated length of the stent to achieve an iFR > 0.89 is 50.6 mm.
Follow-up
Patients will be followed either through phone calls or physical examination at the 3, 6 and 12-month follow-up. At the 6-month follow-up a stress single-photon emission computed tomography (physiological or pharmacological) will be performed in all patients. The composite of cardiovascular death, definitive or probably stent thrombosis, new target lesion failure or new target lesion revascularization will be considered as MACE.
Quantitative coronary measurements
Quantitative coronary measurements will be performed using a validated system (CAAS system, Pie Medica Imaging, Netherlands). The measurements analyzed will be the vessel reference diameter, the vessel minimal lumen diameter, and the percentage of stenosis. All measurements will be taken at baseline and after the PCI.
Statistical analysis
Regarding the statistical analysis, quantitative variables will be expressed as mean ± standard deviation and qualitative variables as absolute numbers and percentages. To determine the relationship among quantitative variables, we will be using the paired Student t test for paired data. To determine the relationship among the qualitative ones, we will use the chi-squared test. In all cases, differences will be considered significant with P values < .05. We will be using the IBM SPSS Statistics software package (version 24.0 for Macintosh, SPSS Corp., United States). To calculate the sample size, we have performed a retrospective analysis of the last 20 patients who were treated at our centre and showed a sequential or diffuse lesion in the coronary vessel analyzed from the iFR-pullback study. The mean length of the stent implanted was 43 ± 9 mm and the reduction of stent length was 12 ± 8 mm on the angiographic analysis. With these data, we have stablished an expected length reduction of 15 mm. The calculated sample size to achieve the primary endpoint with an 80% confidence level and a 5% margin of error was 100 patients.
RESULTS
The recruitment of patients started back in February 2020. After 1 month, we have included the first 7 patients. We expect to complete the recruitment by February 2021 and the follow-up by February 2022.
DISCUSSION
To our knowledge, this randomized study is, the first one to assess the potential benefits of using the SyncVision software in long, sequential or diffuse coronary lesions. Currently, the study is in the recruitment phase and the first patients have already been recruited.
The iFR has proven to be useful in the PCI guide decision-making process.7,8 However, the evidence supporting the use of SyncVision is scarce and controversial in long, sequential or diffuse lesions. On the one hand, the software allows us to know the coronary segments with the highest physiological compromise. This allows us to revascularize only those segments that immediately improve the physiological result with a potential reduction of the length of the stent implanted, which happens to be a predictor of MACE at the follow-up.9 On the other hand, it’s possible that even if we obtain a good immediate physiological result and a reduction of the stent length implanted we won’t be fully covering the plaque in some lesions or coronary segments, which has also proven to be a predictor of MACE.6
A limitation of the study is the sample size, enough to achieve the primary endpoint, but probably inadequate to see differences in MACE. However, we think that it can provide an early insight on the utility of iFR pullback study to guide the PCI decision-making process in this type of lesion. Also, it can be a hypothesis-generator study for future larger-scale studies to show benefits in terms of clinical events reduction.
For these reasons, we believe that the iLARDI is an interesting study that will shows us the potential benefit of SyncVision to guide the PCI decision-making process in long, sequential or diffuse coronary lesions. We intend to complete the results by February 2022.
CONCLUSIONS
The iLARDI study is the first randomized trial to assess the potential utility of SyncVision-guided revascularization in long, sequential and diffuse coronary lesions.
FUNDING
Funds from the Plan Andaluz de Investigación, Desarrollo e Innovación (PAIDI) have been used to pay for the liability insurance associated with clinical research.
AUTHORS' CONTRIBUTION
All the authors have participated in the study and in the manuscript:
F. Hidalgo has participated has mainly drafted of the manuscript and has participated in the conception and design of the study. R. González has also participated in the conception and design of the study, and in the analysis and interpretation of data. S. Ojeda has mainly participated in the conception, design of the study and revision of the manuscript. C. Pericet has participated in the conception and design of the study. A. Lostalo has also collaborated in the analysis and interpretation of data. J. Segura has also revised it critically for important intellectual content. N. Paredes and J.C. Elizalde have also contributed in the analysis and interpretation of data. A. Luque has participated in the draft of the manuscript. F. Mazuelos has also contributed in the analysis and interpretation of data. J. Suárez de Lezo and M. Romero have revised it critically for important intellectual content. M. Pan has done the final approval of the manuscript submitted.
CONFLICTS OF INTEREST
F. Hidalgo, S. Ojeda, and J. Segura received personal fees from Philips Volcano. M. Pan received minor fees from Abbott, Philips Volcano, and Terumo. The remaining authors declared no conflicts of interest whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- The physiological assessment of coronary lesions is a routine practice in the cath lab. The iFR and the SyncVision software allow us to know what is the individual contribution of every coronary lesion and contribute in the PCI decision-making process. However, to our knowledge, no randomized studies have been published on the utility of their use in long, sequential and diffuse coronary lesions.
WHAT DOES THIS STUDY ADD?
- The iLARDI study will show the potential utility of SyncVision/iFR-guided revascularizations in this type of lesions (long, sequential and diffuse coronary lesions) regarding the reduction of the stent length and the potential reduction of major adverse cardiovascular events at the follow-up.
REFERENCES
1. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
2. Kim H-L, Koo B-K, Nam C-W, et al. Clinical and physiological outcomes of fractional flow reserve guided percutaneous coronary intervention in patients with serial stenosis within one coronary artery. JACC Cardiovasc Interv. 2012;5:1013?1018.
3. Nijjer SS, Sen S, Petraco R et al. The Instantaneous Wave-Free Ratio (iFR) pullback:a novel innovation using baseline physiology to optimise coronary angioplasty in tandem lesions. Cardiovasc Revasc Med. 2015;16:167-171.
4. Nijjer SS, Sen S, Petraco R et al. Pre-Angioplasty Instantaneous Wave-Free Ratio Pullback Provides Virtual Intervention and Predicts Hemodynamic Outcome for Serial Lesions and Diffuse Coronary Artery Disease. JACC Cardiovasc Interv. 2014;7:1386-1396.
5. Kikuta Y, Cook CM, Sharp ASP et al. Pre-Angioplasty Instantaneous Wave-Free Ratio Pullback Predicts Hemodynamic Outcome In Humans With Coronary Artery Disease. Primary Results of the International Mul-ticenter iFR GRADIENT Registry. JACC Cardiovasc Interv. 2018;11:757-767.
6. Costa MA, Angiolillo DJ, Tannenbaum M et al. Impact of Stent Deployment Procedural Factors on Long-Term Effectiveness and Safety of Sirolimus-Eluting Stents (Final Results of the Multicenter Prospective STLLR Trial). Am J Cardiol. 2008;101:1704-1711.
7. Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer SS et al. Use of instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376:1824-1834.
8. Gotberg M, Crhistiansen EH, Gudmundsdottir IJ, et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017;376:1813?1823.
9. Coner A, Cicek D, Akinci S, et al. Mid-term clinical outcomes of new generation drug-eluting stents for treatment of diffuse coronary artery ||aadisease. Turk Kardiyol Dern Ars. 2018;46:659-666.
Corresponding author: Servicio de Cardiología, Hospital Universitario Reina Sofía, Avenida Menéndez Pidal 1, 14004 Cordoba, Spain.
E-mail address: fjhl.87@gmail.com (F. Hidalgo Lesmes).











