ABSTRACT
Heart failure (HF) is the leading cause of hospitalization in the Western world. Despite improvements in diagnostic tools and therapies, a substantial number of patients with HF still remain highly symptomatic, with a poor quality of life. Most of these patients are ineligible for heart transplantation or left ventricular assist device placement, which underscores an unmet clinical need in this population. Novel device-based HF therapies represent therapeutic options for these patients to improve their symptoms and quality of life. First-in-man studies showed promising results in terms of feasibility, and device performances. However, there is still scarce data regarding efficacy. In this review, we focus on the pathophysiological rationale, emerging data, concerns, and future perspective behind the 3 most studied type of device-based HF therapy: interatrial shunt devices, designed to decompress the left atrium and prevent pulmonary edema; ventriculoplasty devices, designed to physically restore the left ventricle in patients with maladaptive left ventricular remodeling; and cardiorenal flow modulator devices, designed to improve diuresis and renal function in acute decompensated heart failure with cardiorenal syndrome.
Keywords: Heart failure. Novel devices. Interventional cardiology.
RESUMEN
La insuficiencia cardiaca (IC) es la principal causa de hospitalización en los países desarrollados. A pesar de las mejoras en el diagnóstico y las terapias, una proporción importante de pacientes con IC aún persisten muy sintomáticos o con pobre calidad de vida. La mayoría de estos pacientes, además, no son candidatos a trasplante cardiaco ni a asistencia ventricular de destino. Así pues, existe una necesidad clínica no cubierta de tratar a este creciente subgrupo de pacientes. Los nuevos dispositivos percutáneos para IC son una opción de tratamiento para mejorar los síntomas y la calidad de vida de estos pacientes. Los primeros estudios en humanos con estos dispositivos han mostrado unos resultados prometedores en términos de factibilidad, seguridad e integridad de los dispositivos. No obstante, todavía hay pocos datos sobre su eficacia. En esta revisión nos centramos en describir las características, las ventajas y los inconvenientes, así como las evidencias, de los 3 tipos principales de dispositivos percutáneos para la IC, con especial énfasis en la base fisiopatológica subyacente que justifica su diseño: los dispositivos de derivación interauriculares, que pretenden descomprimir la presión de la aurícula izquierda y así evitar el edema pulmonar; los dispositivos de ventriculoplastia, que restauran físicamente el ventrículo izquierdo en situaciones de mal remodelado ventricular; y los dispositivos de modulación del flujo cardiorrenal, diseñados para mejorar la diuresis en situación de IC aguda descompensada con síndrome cardiorrenal asociado.
Palabras clave: Insuficiencia cardiaca. Nuevas terapias. Cardiología intervencionista.
Abbreviations ADHF: acute decompensated heart failure. GDMT: guideline-directed medical therapies. HF: heart failure. HFrEF: heart failure with reduced ejection fraction. IASD: interatrial shunt device. LV: left ventricle. RV: right ventricle.
INTRODUCTION
Heart failure (HF) is the leading cause of hospitalization in the Western world, and a major issue for public health. The estimated prevalence of HF is between 1% and 2% in the adult population in developed countries, and up to 10% among patients aged > 70 years.1 Despite improvements in diagnostic tools and guideline-directed medical therapies (GDMT) such as continuous monitoring of pulmonary artery pressures, sacubitril, and selective sodium–glu- cose cotransporter 2 inhibitors, a significant proportion of patients with HF remain symptomatic and with a poor quality of life.2-4 According to the latest European data, the annual mortality and re-hospitalization rates in patients with HF are between 7%-17%, and 34%-44% respectively.4 Most of these individuals are ineligible for heart transplantation or left ventricular assist device placement, which underscores an unmet clinical need in this ever growing population. Consequently, novel applications of minimally invasive, device-based therapies are being developed to bridge this treatment gap, that is now starting to fall into an emerging sub-specialty called «interventional heart failure». Novel device-based HF therapies may represent an option to improve the quality of life and reduce the rates of hospitalization or even mortality of these patients who are GDMT-optimized, yet with residual morbidity and suboptimal quality of life.
This article will focus on the pathophysiological rationale and emerging data and concerns behind some of these device-based HF therapies that have been designed to target a range of mechanisms involved in the HF syndrome.
INTERATRIAL SHUNT DEVICES
An early sign of left ventricular (LV) failure (involving both preserved or reduced ejection fraction) is an increased LV end-diastolic pressure, retrogradely transmitted to the left atrium and pulmonary capillaries causing dyspnea and ultimately pulmonary edema if left untreated.5 Interatrial shunt devices (IASD) create a permanent interatrial communication using a conventional percutaneous transseptal approach. It is intended to dynamically decompress left atrial pressure, and thus, attenuate or even reverse the underlying mechanism of pulmonary edema.6 However, left-to-right interatrial shunt may increase right ventricular (RV) preload and eventually RV dilatation. Prior studies suggest that the size of the shunt plays a key role in the final outcomes. Indeed, the ideal size of the shunt should allow the reduction of left atrial pressure without hampering right heart function: too large IASDs may increase the Qp/Qs ratio enough to cause RV failure while too small IASDs may have negligible hemodynamic and clinical effects. Data from first-in-man studies indicate that devices < 10 mm are unlikely to cause hemodynamically significant shunting (ie, Qp/Qs ratio > 1.5) or RV size/functional compromise.7 The 2 shunt devices most studied to date are the 5 mm- (V-Wave device, V-Wave Ltd., Israel) and 8 mm-long (Interatrial Shunt Device, Corvia Medical, United States) in diameter apertures (figura 1A, figura 1B and table 1).8 Another concern is related to patients with stiff or fibrotic atria or RV. In this scenario the right atrium or RV may not be able to accept an increased preload. For all these reasons, patients with restrictive cardiomyopathy, pulmonary hypertension (pulmonary vascular resistance > 4 Wood units) or RV dysfunction have been excluded from shunt studies. Finally, paradoxically, strokes due to transient flow reversal are another potential concern when using this technology.
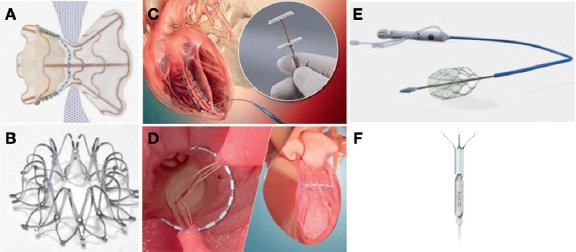
Figure 1. Interventional heart failure therapy devices. A: V-Wave (V-Wave Ltd., Israel) is an interatrial shunt device with an hourglass-shaped self-expandable nitinol frame and expanded PTFE skirt with 5 mm central hole. B: Interatrial Shunt Device (Corvia Medical, United States) provides 8 mm of central hole. C: AccuCinch ventricular restoration system (Ancora Heart, United States) is a fully percutaneous left ventriculoplasty device. D: Revivent TC System (BioVentrix Inc., United States) is a left ventriculoplasty device that uses micro-anchors to exclude a scar via hybrid approach (jugular plus mini lateral thoracotomy). E: Doraya catheter (Revamp Medical, Israel) reduces both the venous renal hypertension and preload to improve diuresis in acute decompensated heart failure (ADHF) with cardiorenal syndrome (CRS). F: Aortix (Procyrion Inc., United States) device is a pump that improves the renal arterial perfusion pressure and reduces the left ventricular afterload. Aortix is intended to improve diuresis in ADHF with CRS.
Table 1. Device mechanism, features and, evidence of interventional heart failure devices
| Device | Device mechanism & features | Trial | Study design | Main inclusion criteria | N | Main results |
|---|---|---|---|---|---|---|
| V-Wave | – Interatrial shunt (transseptal approach). – Fully percutaneous (via femoral vein using a 12-Fr delivery system). – Hourglass-shaped device on a nitinol frame with an expanded PTFE skirt. – Lumen diameter: 5 mm. – Second generation has no unidirectional valve to ensure left-to-right shunt | VW-SP-1 + Canadian cohort8 | Multicenter, single-arm, open-label, phase I trial with a 12-month follow-up | – NYHA class III-IV; ≥ 1 HF hospitalization within the last year or ↑ BNP | 38 (30 with HFrEF) | – 1-year rate of MACE: 2.6% (1 tamponade) – Significant improvements in NYHA class, QoL, KCCQ – Significant increase in the Qp/Qs ratio |
| RELIEVE-HF NCT03499236 | Multicenter, sham-controlled, blinded RCT with a 1:1 allocation ratio, and a 1-to-2-year follow-up | – NYHA class II-IV; ≥ 1 HF hospitalization within the last year or ↑ BNP PCWP > RAP; PVR < 4 WU; transseptal eilgible | 500 (ongoing) | Enrolling (estimated completion by 2022) – Endpoints: device MDAE, MACE, NYHA, KCCQ, and 6MWT | ||
| Interatrial Shunt Device | – Interatrial shunt (transseptal approach) – Fully percutaneous (femoral vein) – Nitinol, self-expanding metal cage with a double-disc design and an opening (barrel) in the center – Central hole of 8 mm | REDUCE LAP-HF NCT01913613 | Multicenter, open-label, single-arm with a 6-month follow-up | – NYHA class II-IV; LVEF > 40%; PCWP ≥ 15 mmHg (or 25 mmHg exercise) | 64 | – No MACE; 52% had a reduction in resting PCWP; 54% had a reduction in PCWP during exertion; improvement in NYHA class; QoL, and 6MWT |
| REDUCE LAP-HF I NCT02600234 | Multicenter, double-blind, sham-controlled RCT with a 1:1 allocation ratio, and a 1-year follow-up | – NYHA class III-IV; LVEF > 40%; Exercise PCWP ≥ 25 mmHg; PCWP-RAP ≥ 5 mmHg; prior HF hospitalization or ↑BNP | 44 | – Reduction in PCWP on exercise; similar rate of MACCE, and no strokes in either one of the 2 arms. – Trends of reduction of HF-related hospitalizations; improvement in QoL, and RV size in the device arm | ||
| Revivent TC System | – Ventriculoplasty – Hybrid (jugular vein + mini lateral thoracotomy) – Anchors and external locking on the LV epicardial surface | REVIVE-HF NCT03845127 | Multicenter prospective RCT with a 6-month follow-up (2:1 allocation ratio; device vs guideline-directed medical therapy) | – HF symptoms with previous myocardial infarctio, increased LV systolic volume, and contiguous scar located in the anterior/ apical LV | 180 (ongoing) | Enrolling (estimated completion by 2022). – Endpoints: 6MWT distance at 6 months; QoL at 6 months; change in NYHA class at 6 months; LVESVI at 6 months; LVEF at 6 months |
| AccuCinch Ventricular Restoration System | – Ventriculoplasty – Fully percutaneous; femoral artery access, retrograde aortic approach; initially designed for mitral regurgitation – Cinching anchors attached to the mitral subvalvular apparatus | CORCINCH-HF NCT04331769 | Multicenter, open-label, RCT with a 5-year follow-up. | – NYHA class II-IV; LVEF, 20% to 40%; and LVEDD > 55 mm; 6MWT distance, 100 m to 450 m | 400 (ongoing) | Enrolling (estimated completion by 2025) – Endpoints: MAEs at 6 months, and 1 year; changes in the KCCQ score; change in the 6MWT; composite of all-cause mortality, LVAD implant or heart transplantation, HF hospitalizations, and changes in the KCCQ score |
| Papillary muscle sling | – Ventriculoplasty – Femoral artery access, retrograde aortic approach – 4 mm PFTE graft implanted around the base of the papillary muscles and then tightened | NCT04475315 | Single-center, open-label RCT (1:1 allocation ratio; CABG vs CABG + sling) with a 5-year follow-up | – NYHA class II-IV; LVEDD ≥ 55; LVEF 20% to 40%; FMR ≤ 2+; end-systolic interpapillary muscle distance ≥ 20 mm; ischemic or nonischemic cardiomyopathy | 40 (ongoing) | Enrolling (estimated completion by 2026) – Endpoints: changes of LVEF and LV volume, mortality, MACE, functional mitral regurgitation severity, change in QoL and the 6MWT, all-cause readmission, HF readmission, and in the rate of mitral leaflet tenting area |
| Doraya catheter | – Venous renal flow modulator via femoral vein (12-Fr delivery system) – Decreased renal hypertension and RV preload | NCT03234647 | Multicenter, first-in-man, single-group study of feasibility, and safety | – ADHF with poor diuretic response | 9 | Enrolling ended back in May 2021. – Device-related or procedural serious adverse events at 60 days |
| Aortix | – Arterial renal flow modulator via femoral artery (18-Fr delivery system) – Pump that increases aortic flow (up to 5 L/min), and renal perfusion pressure, and reduces the LV afterload | NCT04145635 | Multicenter, prospective non-RCT of feasibility and safety | – ADHF with HFrEF or HFpEF – Worsening renal function after 48 hours of IV diuretics (increase of 0.3 mg/dL) – Persistent congestion (PCWP ≥ 20 or central venous pressure ≥ 12mmHg) | 60 (ongoing) | Enrolling (estimated completion by 2022) – Endpoints: 30-day serious adverse events, serious procedural adverse events, device performance, 7-day decrease of central venous pressure or PCWP > 20%, changes in urine output, and lower BNP levels by 20% |
6MWT, 6-minute walk test; ADHF, acute decompensated heart failure; BNP, brain natriuretic peptide; CABG, coronary artery bypass graft; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; IV, intravenous; KCCQ, Kansas City Cardiomyopathy Questionnaire; LV, left ventricle; LVAD, left ventricular assist device; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end-systolic volume index; MACE, major adverse cardiovascular event; MACCE, major adverse cardiovascular and cerebrovascular event; MAE, major adverse event; MDAE, medical device adverse event; NYHA, New York Heart Association; PCWP, pulmonary capillary wedge pressure; PVP, pulmonary vascular resistance; QoL, quality of life; RAP, right atrial pressure; RCT, randomized clinical trial; RV, right ventricle. | ||||||
The first-in-man experience with both the V-wave and the Corvia Medical devices showed significant improvements in quality of life, HF symptom relief, and exercise capacity.6,8-9 There are currently several ongoing studies, and randomized clinical trials assessing IASD in patients with symptomatic HF despite GDMT including both HF with reduced or preserved LV function patients (table 1, REDUCE LAP-HFREF trial [NCT03093961], REDUCE LAP-HF trial II [NCT03088033], and REDUCE LAP-HF IV [NCT04632160]).
VENTRICULOPLASTY (LEFT VENTRICULAR RESTORATION)
The LV has a unique architecture with 3 different myofiber orientations and an elongated ellipsoid shape that are essential for its optimal function. Different pathological states cause molecular and cellular changes that alter the obliquity of the myofibers, which become more horizontal, and macroscopically result in chamber dilatation and increased sphericity. This early adaptive response is ultimately detrimental and self-propagating, resulting in a loss of ventricular function (maladaptive remodeling). Maladaptive LV remodeling is clearly associated with poor prognosis.10
Ventriculoplasty refers to a physical intervention aimed to anatomically modify the LV geometry. The rationale is based on Laplace’s Law, according to which wall tension and LV wall stress are decreased by reductions in the LV radius, thus reversing or attenuating maladaptive LV remodeling. Compared to the ejection fraction, LV volume reduction is likely to be equally important in improving symptoms and possibly clinical outcomes. Prior surgical attempts of this concept showed disappointing results due to protocol deviations, and poor patient selection, thus contributing to less than anticipated LV volume reductions compared to the control arm. However, a post-hoc analysis demonstrated promising results (significant and sustained mortality reductions out to 6 years) in patients with an achieved LV end-systolic volume index < 70 mL/m2 with strong trends in survival advantage in patients who achieved a 30% threshold in LV end-systolic volume index reduction.11 Despite the controversial results of surgical LV reconstruction, there is considerable interest in percutaneous reverse LV remodeling, specifically in patients with HF with reduced ejection fraction (HFrEF). Several methods have been developed to perform percutaneous left ventriculoplasties. However, we will be focusing on the 3 methods that showed promising results in early feasibility studies: the Revivent TC system (BioVentrix Inc., United States), the AccuCinch (Ancora Heart, United States), and the papillary muscle sling.
The BioVentrix Revivent TC system is a hybrid transcatheter procedure via mini lateral thoracotomy and transcatheter jugular access. The system is designed to exclude an aneurysm or scar located in the anterior or apical LV wall. A hinged anchor is deployed inside the RV side of the distal interventricular septum (via jugular access) and 1 external locking anchor on the LV epicardial surface (via minithoracotomy). A tether is used to draw the 2 anchors towards each other until enough contact between the 2 opposing walls has been achieved. This action is repeated along the long axis of the LV, resulting in the exclusion of the dysfunctional scar tissue from a healthy and functional myocardium (figura 1C). Data from a study of 86 patients demonstrated improvements in the LV ejection fraction, LV volumes, quality of life, and functional status.12 Currently, there are 2 pivotal trials assessing this therapy (the REVIVE-HF [NCT03845127], and the ALIVE [NCT02931240]) (table 1).
The AccuCinch ventricular restoration system consists of a predesigned tracking catheter that, via retrograde aortic access, is positioned to encircle the basal aspect of the LV, which is used to position a band anchored to the basal LV myocardium. Tension is applied to a cable reducing the basal wall diameters and the LV volumes13 (figura 1D). Although this device was initially designed to treat functional mitral regurgitation, the current focus remains on patients with HFrEF. There is currently an ongoing pivotal randomized clinical trial assessing this device in individuals with HFrEF vs GDMT (table 1, CORCINCH-HF [NCT04331769]).
The papillary muscle sling procedure, based on an existing surgical sling procedure, is aimed at reducing the lateral interpapillary muscle separation distance. A sling is used to draw together the LV via retrograde aortic access. Currently, there is 1 clinical trial assessing this technique in a surgical cohort (table 1, NCT04475315).
CARDIORENAL FLOW MODULATION
Acute decompensated HF (ADHF) in patients with prior renal injury and/or cardiorenal syndrome is an extremely challenging scenario for medical management. The pathophysiology of cardiorenal syndrome is complex, dynamic, and multifactorial, including hemodynamic and neurohumoral axis alterations.14 Data from previous studies suggest that the difference between renal arterial driving pressure and venous outflow pressures must remain sufficiently large for the proper renal blood flow and glomerular filtration. Maintenance of this homeostatic mechanism is especially important in patients with preexisting renal injuries. In patients with ADHF and cardiorenal syndrome, both pre-renal perfusion reduction, and renal venous hypertension are present. Renal venous hypertension increases renal resistance, which in turn impairs intrarenal blood flow. The decrease in renal perfusion is aggravated by the preglomerular vasoconstriction caused by the neurohumoral activation of the renin-angiotensin-aldosterone axis, which results in increased proximal tubular sodium and water reabsorption to maintain an effective plasma volume. This results in oliguria, worsening congestion symptoms, and diuretic resistance. The relationship between diuretic resistance and poor outcomes in this scenario is well established.14
There are 2 main types of devices designed to interrupt the vicious circle of cardiorenal syndrome in ADHF: those aimed at reducing renal venous hypertension such as the Doraya catheter (Revamp Medical, Israel); and those aimed at increasing arterial renal perfusion pressure such as the Aortix (Procyrion Inc., United States) and the Second Heart Assist (Second Heart LLC, United States) devices.
The Doraya catheter is a self-expanding flexible nitinol frame covered in its distal portion to restrict blood flow. It is placed in the inferior vena cava below the renal vein via femoral vein access using a 12-Fr delivery system. It serves as a temporary flow regulator for up to 24 hours. Doraya causes a temporal decrease in central venous pressure at the renal vein level, thus reducing cardiac preload, and contributing to LV unloading15 (figura 1E). A first-in-man clinical study to assess the safety, feasibility, and hemodynamic effects of this device in patients with ADHF, congestion, and an inadequate response to diuretics completed enrollment back in May 2021 (table 1, NCT03234647).
Aortix is a percutaneous axial pump positioned in the suprarenal descending aorta via transfemoral approach using an 18-Fr delivery system. This device increases aortic flow, decreases afterload, and can provide up to 5 L/min (figura 1F). A study performed in the percutaneous coronary intervention setting demonstrated a 10-fold increase in urine output.16 There is an ongoing study aimed at evaluating the feasibility, safety, and efficacy profile of Aortix in patients with ADHF (HF with preserved ejection fraction, and HRrEF), and cardiorenal syndrome (table 1, NCT04145635).
Despite improvements made in the management of HF, a substantial proportion of patients still remain severely symptomatic, and with a poor quality of life. Interventional HF is a promising new field within interventional cardiology to provide a percutaneous device-based therapeutic response to these patients. Interatrial shunts, percutaneous ventriculoplasties, and cardiorenal flow modulators are some of the most remarkable devices in this emerging field.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
The authors have contributed equally in all the writing/design phases of this manuscript.
CONFLICTS OF INTEREST
None declared.
REFERENCES
1. Ponikowski P, Voors AA, Anker SD, et al.;ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129-2200.
2. Bayes-Genis A, Codina P, Abdul-Jawad Altisent O, et al. Advanced remote care for heart failure in times of COVID-19 using an implantable pulmonary artery pressure sensor:the new normal. Eur Heart J Suppl. 2020;22:P29-P32.
3. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375:323-334.
4. Maggioni AP, Dahlström U, Filippatos G, et al. EURObservationalResearch Programme:regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013;15:808-817.
5. Ritzema J, Troughton R, Melton I, et al. Physician-Directed Patient Self-Management of Left Atrial Pressure in Advanced Chronic Heart Failure. Circulation. 2010;121:1086-1095.
6. Del Trigo M, Bergeron S, Bernier M, et al. Unidirectional left-to-right interatrial shunting for treatment of patients with heart failure with reduced ejection fraction:a safety and proof-of-principle cohort study. Lancet. 2016;387:1290-1297.
7. Kaye DM, HasenfußG, Neuzil P, et al. One-Year Outcomes After Transcatheter Insertion of an Interatrial Shunt Device for the Management of Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2016;9:e003662.
8. Rodés-Cabau J, Bernier M, Amat-Santos IJ, et al. Interatrial Shunting for Heart Failure:Early and Late Results From the First-in-Human Experience With the V-Wave System. JACC Cardiovasc Interv. 2018;11:2300-2310.
9. Feldman T, Mauri L, Kahwash R, et al. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure With Preserved Ejection Fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]). Circulation. 2018;137:364-375.
10. Adhyapak SM, Parachuri VR. Architecture of the left ventricle:insights for optimal surgical ventricular restoration. Heart Fail Rev. 2009;15:73-83.
11. Michler RE, Rouleau JL, Al-Khalidi HR, et al. Insights from the STICH trial:change in left ventricular size after coronary artery bypass grafting with and without surgical ventricular reconstruction. J Thorac Cardiovasc Surg. 2013;146:1139-1145.e6.
12. Klein P, Anker SD, Wechsler A, et al. Less invasive ventricular reconstruction for ischaemic heart failure. Eur J Heart Fail. 2019;21:1638-1650.
13. Gooley RP, Meredith IT. The Accucinch transcatheter direct mitral valve annuloplasty system. EuroIntervention. 2015;11(Suppl W):W60-1.
14. Rangaswami J, Bhalla V, Blair JEA, et al. Cardiorenal Syndrome:Classification, Pathophysiology, Diagnosis, and Treatment Strategies:A Scientific Statement From the American Heart Association. Circulation. 2019;139:e840-e878.
15. Dierckx R, Vanderheyden M, Heggermont W, Goethals M, Verstreken S, Bartunek J. Treatment of Diuretic Resistance with a Novel Percutaneous Blood Flow Regulator:Concept and Initial Experience. J Card Fail. 2019;25:932-934.
16. Vora AN, Schuyler Jones W, DeVore AD, Ebner A, Clifton W, Patel MR. First-in-human experience with Aortix intraaortic pump. Catheter Cardiovasc Interv. 2019;93:428-433.
* Corresponding author: Interventional Cardiology Section, Department of Cardiovascular Medicine, 9500 Euclid Ave (J2-3), Cleveland Clinic, Cleveland, OH 44195, United States.
E-mail address: purir@ccf.org (R. Puri).











