ABSTRACT
Introduction and objectives: The safety of physiology-based revascularization in patients with diabetes mellitus has been scarcely investigated. Our objective was to determine the safety of deferring revascularization based on the fractional flow reserve (FFR) or the instantaneous wave-free ratio (iFR) in diabetic patients.
Methods: Single-center, retrospective analysis of patients with intermediate coronary stenoses in whom revascularization was deferred based on FFR > 0.80 or iFR > 0.89 values. The long-term rate of major adverse cardiovascular events, a composite of all-cause mortality, myocardial infarction, and target vessel revascularization (TVR), was assessed in diabetic and non-diabetic patients at the follow-up. The rate of TVR based on the type of physiological index used to defer the lesion was also evaluated.
Results: We evaluated 164 diabetic (214 vessels) and 280 non-diabetic patients (379 vessels). No significant differences in the rate of major adverse cardiovascular events was seen between diabetic and non-diabetic patients (20.1% vs 13.2%; P = .245) at a median follow-up of 43 months. All-cause mortality and cardiac death were not statistically different between both groups in the adjusted analysis (P > .05). A trend towards a higher rate of myocardial infarction was seen in diabetic patients (6.7% vs 2.9%; P = .063). However, the rate of target vessel myocardial infarction was similar in both groups (P = .874). Overall, TVR was similar in diabetics and non-diabetics (4.7% vs 4.2%; P = .814); however, when analyzed based on the physiological index, numerically, diabetics had a higher rate of TVR when the FFR was used in the decision-making process compared to when the iFR was used (6.4% vs 0.0%; P = .064).
Conclusions: Deferring the revascularization of intermediate stenoses in patients with DM based on the FFR or the iFR is safe regarding the risk of TVR or target vessel myocardial infarction, with a rate of events at the long-term follow-up similar to that seen in non-diabetic patients.
Keywords: Fractional flow reserve. Instantaneous wave-free ratio. iFR. Diabetes mellitus.
RESUMEN
Introducción y objetivos: La seguridad de la revascularización fisiológica en pacientes diabéticos ha sido poco investigada. El objetivo fue determinar la seguridad de diferir la revascularización basándose en la reserva fraccional de flujo (FFR) o en el índice instantáneo libre de ondas (iFR) en pacientes con diabetes mellitus.
Métodos: Análisis retrospectivo, unicéntrico, de pacientes con estenosis coronarias intermedias en quienes se había diferido la revascularización en función de unos valores de FFR > 0,80 o de iFR > 0,89. Se analizó la incidencia a largo plazo de eventos cardiovasculares adversos mayores, una combinación de muerte por cualquier causa, infarto miocárdico y revascularización del vaso diana (RVD) en pacientes con y sin diabetes. También se evaluó la incidencia de RVD según el tipo de índice fisiológico utilizado para diferir la revascularización.
Resultados: Se evaluaron 164 pacientes diabéticos (214 vasos) y 280 pacientes no diabéticos (379 vasos), con una mediana de seguimiento de 43 meses. No se observaron diferencias significativas en los eventos cardiovasculares adversos mayores entre pacientes con y sin diabetes mellitus (20,1 frente a 13,2%; p = 0,245). La mortalidad por cualquier causa y de causa cardiaca no fue estadísticamente diferente entre ambos grupos en el análisis ajustado (p > 0,05). Se observó una tendencia a una mayor incidencia de infarto de miocardio en los pacientes con diabetes mellitus (6,7 frente a 2,9%; p = 0,063), pero el infarto relacionado con el vaso diana fue similar en ambos grupos (p = 0,906). En general, la RVD fue similar en diabéticos y no diabéticos (4,7 frente a 4,2%; p = 0,787); sin embargo, cuando se analizó según el índice fisiológico, los diabéticos tuvieron una mayor tasa numérica de RVD cuando se utilizó la FFR en la toma de decisiones en comparación con el iFR (6,4 frente a 0,0%; p = 0,064).
Conclusiones: Diferir la revascularización de estenosis intermedias en pacientes con diabetes mellitus según la FFR o el iFR es seguro en términos de RVD e infarto relacionado con el vaso diana, con una tasa de eventos en el seguimiento a largo plazo similar a la observada en pacientes sin diabetes mellitus.
Palabras clave: Reserva fraccional de flujo. Indice instantaneo libre de ondas. iFR. Diabetes mellitus.
Abbreviations DM: diabetes mellitus. FFR: fractional flow reserve. iFR: instantaneous wave-free ratio. MACE: major adverse cardiovascular events. TVR: target vessel revascularization.
INTRODUCTION
Physiological evaluation has a class IA recommendation to guide coronary revascularization in the current clinical practice guidelines.1 Fractional flow reserve (FFR) and instantaneous wave-free ratio (iFR) have proven to be safe tools to guide revascularization therapy in several clinical scenarios.2-4
The results of the DEFER trial at the 15-year follow-up showed the long-term safety of FFR to defer therapy in functionally non-significant stenosis.5 Afterwards, the DEFINE-FLAIR and the iFR-SWEDEHEART trials proved the non-inferiority of the iFR compared to the FFR to guide revascularization of moderate stenosis at the 1-year follow-up.3,6 The utility of physiological guidance to guide revascularization in multivessel disease has been confirmed in the FAME and the SYNTAX II clinical trials.7,8
However, the prognostic value of pressure guidewire assessment in certain high-risk groups has not been firmly established yet. A pooled analysis of the DEFINE-FLAIR and the iFR-SWEDEHEART trials found a higher rate of events in patients with acute coronary syndrome in whom revascularization of non-culprit vessels was deferred based on the FFR or the iFR compared to stable patients.3,6 Patients with diabetes mellitus (DM) are a high-risk group with a well-known higher burden of cardiovascular disease and worse prognosis including more extensive atherosclerosis, more prevalence of multivessel disease, and a faster disease progression compared to non-diabetic patients.9-11 The special characteristics of the extent and spread of atherosclerosis in patients with DM raises concerns on the safety surrounding deferring revascularization in this population. Our objective was to evaluate the safety of revascularization deferral based on pressure guidewire interrogation in diabetic patients at the long-term follow-up.
METHODS
Study population
This is a single center, retrospective, and open-label trial. The study population was recruited from a total of 1321 consecutive patients with coronary artery disease in whom the iFR or the FFR indices were used to determine the need for coronary revascularization from January 2012 through December 2016. In 444 patients (34%) the revascularization of ≥ 1 lesions was deferred based on FFR values > 0.80 or iFR values > 0.89. Patients with stable angina and acute coronary syndrome (with non-culprit stenosis interrogated with pressure guidewires) were included in the study. For the analysis, the overall population was divided into 2 groups: DM and non-DM. The DM group was defined based on their past personal history included in their medical records. The study flow chart depicting patient selection is shown on figure 1.
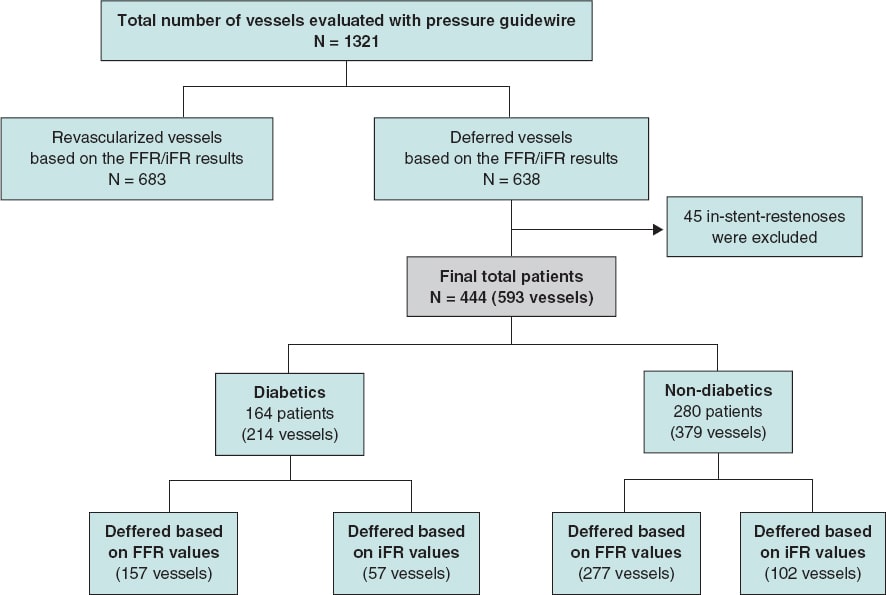
Figure 1. Study flow chart. iFR, instantaneous wave-free ratio; FFR, fractional flow reserve.
This retrospective, cohort study was conducted according to the principles established by the Declaration of Helsinki. Both the informed consent and the research committee assessment were spared due to the retrospective nature of the study; each patient included in the database was encrypted and de-identified to protect everyone’s privacy.
The physiological procedure
Pressure guidewire assessment was performed using a commercial guidewire (Verrata, Philips Healthcare, United States; PressureWire [Certus. Aeris, X] St. Jude Medical, United States) and the standard technique previously reported.3,12 As a standard practice, an intracoronary bolus of nitrates (200 mcg) was administered before the FFR or iFR measurements. The cases submitted for FFR assessment received IV adenosine at a rate of 140 µg/kg/min. The cut-off values to defer revascularization were FFR > 0.80 or iFR > 0.89. The presence of significant drift was discarded by placing the sensor of the pressure guidewire on the tip of the guiding catheter at the end of the physiological measurements acquisition.
In patients with stable angina, the physiological evaluation was performed as part of the same procedure, and all intermediate stenoses were assessed. In patients with acute coronary syndrome, interrogation with the pressure guidewire was performed at a staged procedure in non-culprit vessels only.
Endpoints
The primary endpoint was the 4-year risk of major adverse cardiovascular events (MACE) defined as a composite endpoint of all-cause mortality, myocardial infarction or unplanned target vessel revascularization (TVR). The secondary endpoints were a) the individual components of MACE, b) the rate of target vessel myocardial infarction, and c) the rate of unplanned TVR based on the physiological index used (FFR or iFR)
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD). Discrete variables are summarized as frequency (percentages). Under baseline conditions, group comparisons were made using the Student t test or the Mann Whittney U test for continuous variables and Pearson’s chi-square test for discrete data.
Time-to-event analysis was performed using the Kaplan-Meier method, and group comparison was performed using the Mantel-Cox (log-rank) test. For the primary and secondary endpoint comparison between diabetics and non-diabetics, a Cox Proportional hazards model was used to estimate hazard ratios (HR). The adjusted analysis was performed based on age, sex, hypertension, dyslipidemia, smoking habit, chronic kidney disease, previous stroke, previous percutaneous coronary intervention, and coronary artery bypass graft surgery.
All probability values were 2-sided with 95% confidence intervals (95%CI). P values < .05 were considered statistically significant. The SPSS version 23.0 (IBM Corp, Armonk, NY, United States) and STATA version 15 (Stata Corp, College Station, TX, United States) statistical packages were used for statistical analyses.
RESULTS
Baseline clinical and angiographic characteristics
The baseline clinical characteristics are shown on table 1. In the overall study population, mean age was 68.4 years, and 39.6% were patients with DM (164 patients). As expected, patients with DM had more cardiovascular risk factors and comorbidities compared to patients without DM. There were no significant differences in the clinical presentation between both study groups (P > .05). Most patients received optimal medical treatment at the hospital discharge without significant differences between DM and non-DM patients (P > .05).
Table 1. Baseline characteristics
| Total (N = 444) | Diabetics (N = 164) | Non-diabetics (N = 280) | P | |
|---|---|---|---|---|
| Clinical characteristics, N (%) | ||||
| Sex | .026 | |||
| Male | 340 (76.5) | 116 (70.7) | 224 (80.0) | |
| Female | 104 (23.4) | 48 (29.3) | 56 (20.0) | |
| Age (year) | 68.41 | 70.02 | 67.46 | .003 |
| Arterial hypertension | 321 (72.3) | 138 (84.1) | 183 (65.4) | <.001 |
| Hyperlipidemia | 287 (64.6) | 124 (75.6) | 163 (58.2) | <.001 |
| Current smoker | 253 (57.0) | 85 (51.8) | 168 (60.0) | .093 |
| Chronic kidney disease | 41 (9.2) | 29 (17.7) | 12 (4.3) | .000 |
| COPD | 30 (6.8) | 9 (5.5) | 21 (7.5) | .415 |
| Previous cerebrovascular disease | 21 (4.7) | 12 (7.3) | 9 (3.2) | .049 |
| Peripheral vascular disease | 38 (8.6) | 18 (11.0) | 20 (7.1) | .164 |
| Previous AMI | 47 (10.6) | 17 (10.4) | 30 (10.7) | .908 |
| Previous PCI | 220 (49.5) | 70 (42.7) | 150 (53.6) | .027 |
| Previous CABG | 13 (2.9) | 9 (5.5) | 4 (1.4) | .019 |
| Clinical presentation, N (%) | ||||
| Myocardial infarction | 148 (33.3) | 46 (28.0) | 102 (36.4) | .302 |
| Unstable angina | 89 (20.0) | 33 (20.1) | 56 (20.0) | |
| Stable angina | 112 (25.2) | 44 (26.8) | 68 (24.3) | |
| Silent ischemia | 46 (10.4) | 22 (13.4) | 24 (8.6) | |
| Other | 49 (11.0) | 19 (11.6) | 30 (10.7) | |
| Therapy at discharge, N (%) | ||||
| Aspirina | 408 (93.8) | 150 (94.3) | 258 (93.5) | .720 |
| Clopidogrela | 165 (37.9) | 53 (33.3) | 112 (40.6) | .134 |
| Prasugrela | 22 (5.1) | 9 (5.7) | 13 (4.7) | .663 |
| Ticagrelora | 78 (17.9) | 30 (18.9) | 48 (17.4) | .699 |
| DAPT | 332 (56.3) | 111 (52.6) | 221 (58.3) | .181 |
| Statinsb | 396 (93.2) | 148 (93.1) | 248 (93.2) | .952 |
| Beta-blockersb | 334 (78.6) | 121 (76.1) | 213 (80.1) | .334 |
| ACEIb | 324 (76.2) | 126 (79.2) | 198 (74.4) | .260 |
| Acenocoumarola | 41 (9.4) | 18 (11.3) | 23 (8.3) | .304 |
| Insulin | 53 (8.9) | 53 (24.8) | ||
ACEI, angiotensin converting enzyme inhibitors; AMI, acute myocardial infarction; CABG, coronary artery bypass grafting;; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention. a n = 435. b n = 425. | ||||
Characteristics of vessels with deferred revascularization
On average, deferred revascularization was performed in vessels with stenoses of intermediate severity (percent diameter stenosis, 59.73% ± 9.2%). The most frequently interrogated artery was the left anterior descending coronary artery (43.2%). In most patients, only 1 vessel was deferred (72.7%). Nevertheless, in about 4% of patients, revascularization was deferred in 3 vessels within the same procedure.
In our study population, revascularization deferral was based more frequently in the FFR (434 vessels, 73.2%) compared to the iFR (159 vessels, 26.8%). The same ratio applied to patients with DM: revascularization was deferred in 157 vessels (73.4%) based on FFR values compared to 57 vessels (26.6%) based on iFR values. The mean FFR and iFR values of the overall population were 0.87 ± 0.46 and 0.94 ± 0.41, respectively without any significant differences between diabetic and non-diabetic patients (table 2).
Table 2. Characteristics of the deferred arteries
| Total (N = 593) | Diabetics (N = 214) | Non-diabetics (N = 379) | P | |
|---|---|---|---|---|
| Deferred vessel | ||||
| LMCA | 25 (4.2) | 8 (3.7) | 17 (4.5) | .664 |
| LAD | 256 (43.2) | 90 (42.1) | 166 (43.8) | .681 |
| LCX | 173 (29.2) | 59 (27.6) | 114 (30.1) | .519 |
| RCA | 138 (23.3) | 57 (26.6) | 81 (21.4) | .145 |
| Number of deferred vessels per patient* | ||||
| 1 vessel | 323 (72.7) | 122 (74.4) | 201 (71.8) | .475 |
| 2 vessels | 98 (22.1) | 35 (21.3) | 63 (22.5) | |
| 3 vessels | 19 (4.3) | 7 (4.3) | 12 (4.3) | |
| 4 vessels | 4 (0.9) | 4 (1.4) | 0 (0.0) | |
| Coronary physiological parameters | ||||
| Mean FFR | 0.87 ± 0.46 | 0.86 ± 0.41 | 0.87 ± 0.48 | .387 |
| Mean iFR | 0.94 ± 0.41 | 0.94 ± 0.43 | 0.95 ± 0.40 | .091 |
| Deferred based on FFR values | 434 (73.2) | 157 (73.4) | 277 (73.1) | .942 |
| Deferred based on iFR values | 159 (26.8) | 57 (26.6) | 102 (26.9) | .942 |
FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; LAD, left anterior descending coronary artery; LCX, left circumflex artery; LMCA, left main coronary artery; RCA, right coronary artery. * n = 444. | ||||
Clinical outcomes at the long-term follow-up based on the presence of diabetes
The median follow-up was 43 months [interquartile range, 31.1-55.8] without any differences being reported between DM and non-DM patients. The clinical outcomes are shown on table 3. Diabetic patients had higher rates of MACE (33 [20.1%] vs 37 [13.2%] in non-DM patients) although this difference did not reach statistical significance in the adjusted analysis (HR, 0.98, 95%CI; 0.46-2.11, P = .964). The all-cause mortality rate was higher in diabetics (18 [10.8%] vs 15 [5.3%] in non-diabetics), but the rates of cardiovascular death were not statistically different in either group (3.1% vs 2.1%). A trend towards a higher rate of myocardial infarction was seen in patients with DM (6.7% vs 2.9%; P = .063), yet target vessel myocardial infarction was similar in both groups (HR, 0,87; 95%CI, 0.15-4.89, P = .906). Similar rates of unplanned revascularization and TVR were seen between diabetics and non-diabetics (figure 2 and table 3).
Table 3. Clinical events at the 4-year follow-up based on the presence of diabetes
| Diabetics (N = 164) | Non-diabetics (N = 280) | Unadjusted analysis | Fully adjusted analysis* | |||
|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |||
| MACE | 33 (20.1) | 37 (13.2) | 1.58 (0.99-2.53) | .058 | 0.98 (0.46-2.11) | .964 |
| All-cause mortality | 18 (10.8) | 15 (5.3) | 2.10 (1.06-4.17) | .034 | 2.01 (0.92-4.40) | .079 |
| Cardiovascular death | 5 (3.1) | 6 (2.1) | 1.45 (0.44-4.74) | .543 | 0.72 (0.19- 2.76) | .641 |
| Myocardial infarction | 11 (6.7) | 8 (2.9) | 2.54 (1.02-6.32) | .045 | 2.56 (0.95- 6.91) | .063 |
| Unplanned revascularization | 17 (10.4) | 20 (7.1) | 1.53 (0.80-2.93) | .195 | 1.55 (0.77- 3.10 | .219 |
| Diabetic vessels (N = 214) | Non-diabetic vessels (N = 379) | Unadjusted analysis | Fully adjusted analysis* | |||
| HR (95%CI) | P | HR (95%CI) | P | |||
| Target vessel myocardial infarction | 2 (0.9) | 4 (1.1) | 0.96 (0.18-5.23) | .971 | 0.87 (0.15-4.89) | .874 |
| Target vessel revascularization | 10 (4.7) | 16 (4.2) | 1.15 (0.52-2.54) | .767 | 1.14 (0.38-3.42) | .814 |
95%CI, 95% confidence interval; HR, hazard ratio; MACE, mayor adverse cardiovascular events (all-cause mortality, myocardial infarction, target vessel revascularization). * HR and P values are obtained after adjusting the model with different baseline variables (age, hypertension, dyslipidemia, smoking habit, chronic kidney disease, previous percutaneous coronary intervention, and previous coronary artery bypass grafting). | ||||||
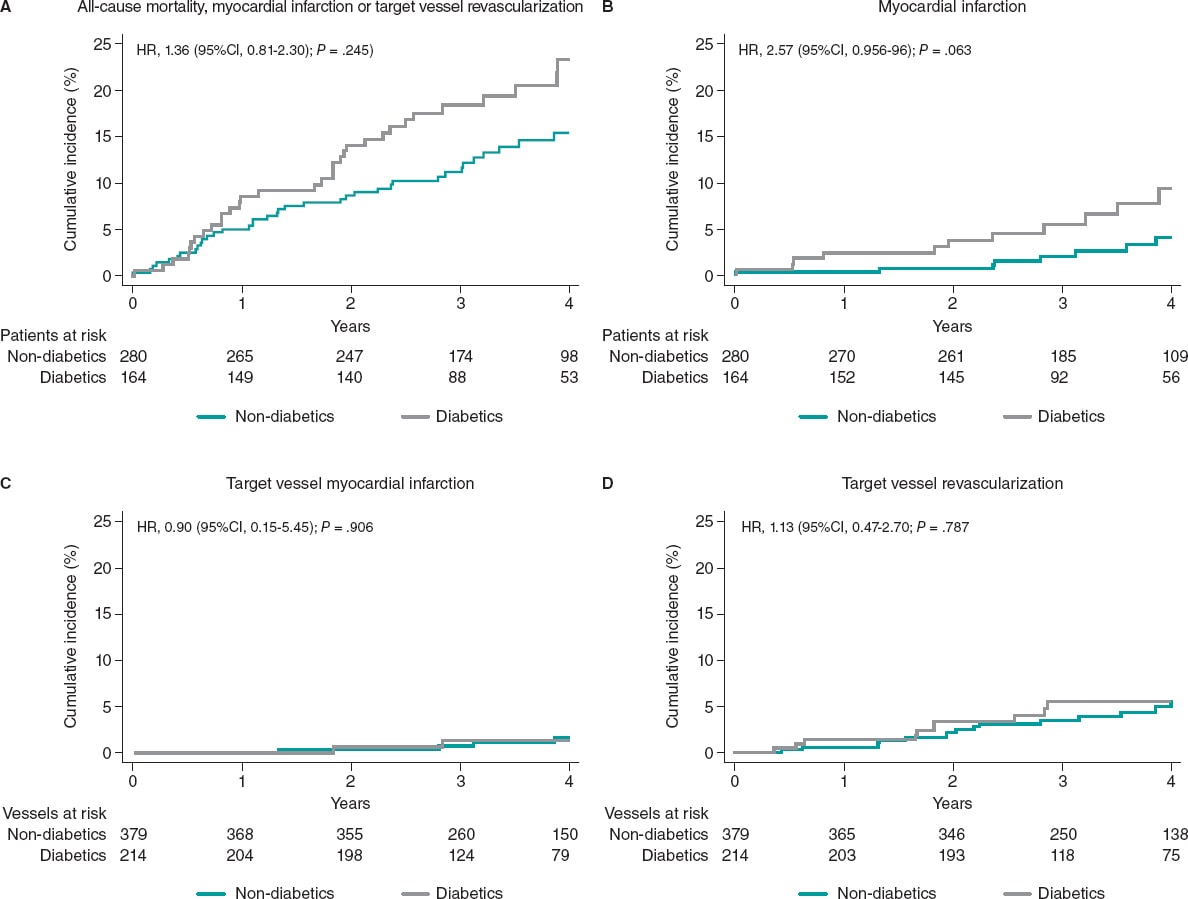
Figure 2. Clinical outcomes in diabetic and non-diabetic patients at the 4-year follow-up. 95%CI, 95% confidence interval; HR, hazard ratio.
Clinical outcomes at the long-term follow-up based on the physiological index used to defer revascularization
In patients with DM the physiological index used (FFR or iFR) that led to revascularization deferral was not associated with a significant difference in the rate of MACE (P = .688) or with significant differences in all-cause mortality, cardiovascular death, myocardial infarction or unplanned revascularization. Similar rates of target vessel myocardial infarction were seen in patients deferred with both techniques (DM or non-DM) (table 4).
Table 4. Clinical outcomes at the 4-year follow-up based on the technique to defer revascularization
| Patients deferred based on FFR values (N = 347) | Patients deferred based on iFR values (N = 97) | P (log-rank) | |
|---|---|---|---|
| MACE | 59 (17.0) | 11 (11.3) | .288 |
| Diabetics | 27 (21.1) | 6 (16.7) | .688 |
| Non-diabetics | 32 (14.6) | 5 (8.2) | .277 |
| All-cause mortality | 25 (7.2) | 8 (8.3) | .574 |
| Diabetics | 13 (10.2) | 5 (13.9) | .417 |
| Non-diabetics | 12 (5.5) | 3 (4.9) | .972 |
| Cardiovascular mortality | 8 (2.3) | 3 (3.1) | .593 |
| Diabetics | 4 (3.1) | 1 (2.8) | .964 |
| Non-diabetics | 4 (1.8) | 2 (3.3) | .436 |
| Myocardial infarction | 16 (4.6) | 3 (3.1) | .762 |
| Diabetics | 10 (7.8) | 1 (2.8) | .396 |
| Non-diabetics | 6 (2.7) | 2 (3.3) | .596 |
| Unplanned revascularization | 33 (9.5) | 4 (4.1) | .133 |
| Diabetics | 16 (12.5) | 1 (2.8) | .112 |
| Non-diabetics | 17 (7.8) | 3 (4.9) | .542 |
| Patients deferred based on FFR values (N = 434) | Patients deferred based on iFR values (N = 159) | P (log-rank) | |
| Target vessel myocardial infarction | 4 (0.9) | 2 (1.3) | .527 |
| Diabetics | 2 (1.3) | 0 (0.0) | .433 |
| Non-diabetics | 2 (0.7) | 2 (2.0) | .172 |
| Target vessel revascularization | 24 (5.5) | 2 (1.3) | .037 |
| Diabetics | 10 (6.4) | 0 (0.0) | .064 |
| Non-diabetics | 14 (5.1) | 2 (2.0) | .244 |
iFR, instantaneous wave-free ratio; FFR, fractional flow reserve; MACE, mayor adverse cardiovascular events (all cause-mortality, myocardial infarction, target vessel revascularization). | |||
The rate of TVR was significantly higher in patients deferred based on FFR values compared to patients deferred based on iFR values (24 [5.5%] vs 2 [1.3%], P = .037). This result was mainly driven by a significant trend towards a higher rate of TVR in patients with DM deferred based on FFR values compared to diabetic patients deferred based on iFR values (10 [6.4%] vs 0 [0%]), a result that did not achieve statistical significance (P = .065). This trend towards a lower rate of TVR in iFR-deferred vessels was not seen in non-DM patients (14 [5.1%] vs 2 [2.0%], P = .244) (figure 3).
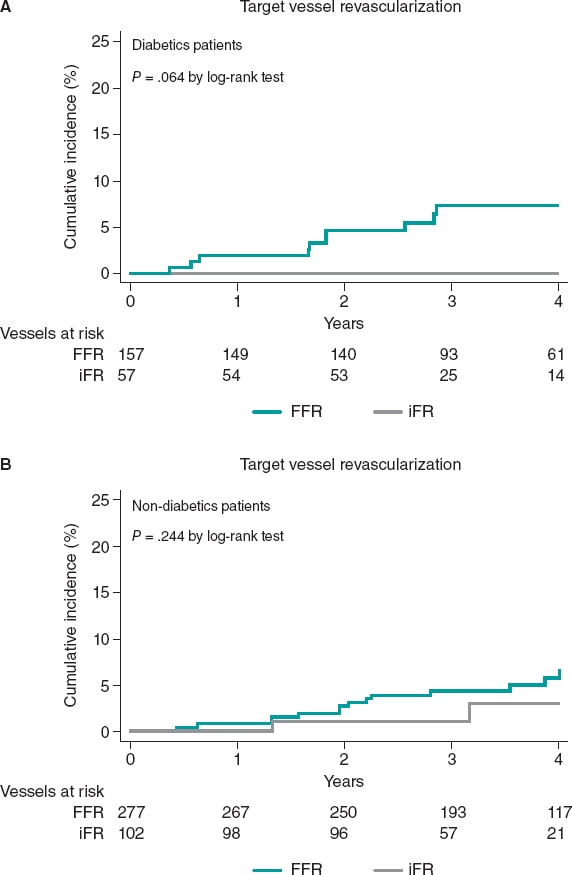
Figure 3. Target vessel revascularization based on the technique used to defer revascularization at the 4-years follow-up. iFR, instantaneous wavefree ratio; FFR, fractional flow reserve.
DISCUSSION
The main findings of this study are: a) patients with DM had high rates of MACE. However, deferring the revascularization of intermediate stenoses in patients with DM based on the physiological assessment results with pressure guidewires is safe regarding the risk of TVR or target vessel myocardial infarction with a similar rate of events at the long-term follow-up compared to that seen in non-diabetic patients; b) both the FFR and the iFR can be used safely to defer intermediate stenosis in diabetic patients. c) there was a trend towards a higher rate of TVR in diabetic patients deffered based on FFR values.
Clinical outcomes based on the presence of diabetes mellitus
The use of coronary physiology to guide revascularization improves patient outcomes compared to angiographic assessment.3,6,7,13 Currently both the FFR and the iFR have a class IA recommendation in the clinical practice guidelines regarding revascularization for the functional assessment of coronary stenoses.1
Diabetic patients are a high-risk population with a more aggressive and accelerated atherosclerosis compared to non-diabetic patients. In the PARADIGM (Progression of atherosclerotic plaque determined by computed tomographic angiography imaging) study, the presence of DM was an independent risk factor for plaque progression.14 In a pooled analysis of 5 intravascular ultrasound trials, Nicholls SJ et al. found that patients with DM had a greater percent atheroma volume and a more rapid progression.15
Around 25% of the patients enrolled in pivotal studies that proved the effectiveness of the FFR and the iFR had DM.3,4,6,16,17 The safety of physiology-guided revascularization deferral in the DM setting has not been specifically assessed in randomized clinical trials. On the other hand, the results of the few non-randomized studies that have evaluated physiology-guided management in diabetics show conflicting results.18,19
Domínguez-Franco et al. analyzed the prognostic safety of the FFR in diabetics. Although, their results are consistent with ours in the sense that no differences were found in the TVR at the long-term follow-up after revascularization deferral in DM vs non-DM patients, the applicability and strength of that study is limited by its small sample size (136 patients, 144 lesions). Also, the use of a FFR cut-off value for the decision-making process was 0.75 while in contemporary practice cut-off values of 0.80 are often used.18
Recently, Kennedy et al. analyzed 250 patients (128 DM, and 122 non-DM patients) and found that DM was associated with a higher rate of failed deferred stenoses (18.1% vs 7.5%, P ≤ .01, Cox regression-adjusted (HR, 3.65; 95%CI, 1.40-9.53, P < .01), and target lesion revascularization of the deferred lesion (17.2% vs 7.5%; HR, 3.52; 95%CI, 1.34-9.30; P = .01). Nevertheless, and consistent with our results, no significant differences in the rate of target vessel myocardial infarction were seen (6.1% vs 2.0%; HR, 3.34; 95%CI, 0.64-17.30; P = 0.15).19 The TLR reported in the former study is much higher than the one seen in our population and the one reported by former studies (eg, in the FAME study the 2-year rate of TLR was 3.2% in FFR-negative lesions).2 These differences can be associated with the characteristics of concomitant medical therapy, which was not specified and may affect the evolution of patients with DM critically. In our study population most patients received optimal medical therapy with over 93% receiving statins while the former study did not specify the medical treatment used. Another important factor can be the percentage of insulin-treated patients with DM (42% in the former study vs 24% in our population). In a different study the same authors found that insulin therapy was a predictive factor of deferred lesion failure in patients with FFR values > 0.80.20 Differences in the risk profile of the populations may, therefore, explain the different results obtained. Our study, with a larger sample size, proves that compared to non-diabetics deferring the revascularization of intermediate stenoses in diabetic patients is safe, and with no differences in TVR at the follow-up. Another study with similar results was the one conducted by Van Belle et al. who saw that the FFR is an important tool to redefine the severity of stenosis in patients with DM with good results at 1 year in deferred patients (HR, 0.77; 95%CI, 0.47-1.25; P = .29; reclassified vs non-reclassified patients with DM).21
Clinical outcomes based on the physiological index used to defer revascularization
Our results suggest that both the FFR and the iFR can be used safely to defer intermediate stenoses in patients with DM. Our findings regarding the low rates of MACE at the follow-up are consistent with the sub-analysis of patients with DM from the DEFINE-FLAIR trial compared to the 1-year follow-up.22 Interestingly enough, we found a trend towards a higher rate of TVR in diabetics deffered with the FFR compared to the iFR. This can be associated with the presence of microvascular dysfunction in diabetic patients, and with the better correlation of iFR with indices that assess microcirculation like coronary flow reserve (CFR). One study evaluated the performance of the iFR and the FFR against invasive CFR in 216 stenoses to find a significantly stronger correlation and a higher diagnostic performance for the iFR (iFR area under the ROC curve, 0.82 vs FFR area under the ROC curve, 0.72; P < .001, for a coronary flow velocity reserve of 2).23 Cook et al. evaluated 567 vessels with sensor-tipped pressure and Doppler ultrasound guidewires and found that with discordant FFR–/iFR+ , the hyperemic flow velocity and the CFR were similar to the FFR+/iFR+ group (P > .05). However, with discordant FFR+/iFR–, the hyperemic flow velocity and the CFR were similar to both the FFR–/iFR– and the coronary unobstructed groups (P > .05).24 These findings may potentially explain the lower performance of FFR in the presence of microvascular dysfunction, and the tendency we found towards a higher TVR in diabetic patients deferred with FFR. Interestingly enough, this tendency was not found in non-DM patients, which supports the hypothesis of microvascular compromise as one of the potential causes for the differences observed between the 2 indices.
Limitations
This study has several limitations. First, this is a single-center observational, retrospective, non-randomized study. The results should be analyzed with caution and can only be interpreted as hypothesis-generating given the small sample size that limits the study statistical power. There were more patients evaluated with the FFR compared to the iFR, which may have influenced the results. Neither clinicians nor patients were blinded to the physiological results, which may have influenced future decisions on revascularization. Most patients had a single deferred vessel, meaning that extrapolation of this data to patients with multivessel disease is complex. Finally, microvascular dysfunction was not evaluated in this population, and the actual impact on the results cannot be determined.
CONCLUSIONS
Deferring the revascularization of intermediate stenoses in patients with DM based on the FFR or the iFR is safe regarding the risk of TVR or target vessel myocardial infarction, with a similar rate of these events at the long-term follow-up compared to the rate seen in non-diabetic patients.
FUNDING
None whatsoever.
AUTHORS’ CONTRIBUTIONS
A.F. Castro-Mejía, and A. Travieso-González contributed to the study idea, design, acquisition, analysis, and interpretation of data, and writing of the article, M.J. Pérez-Vizcayno contributed to both the analysis and interpretation of data, H. Mejía-Rentería, I.J. Núñez-Gil, P. Salinas, L. Nombela-Franco, P. Jiménez-Quevedo, A. Fernández-Ortiz, and C. Macaya contributed to the writing of the article, and made a critical review of its intellectual content. J. Escaned, and N. Gonzalo contributed to the writing of the article, made a critical review of its intellectual content, and gave their final approval to the version that would eventually be published.
CONFLICTS OF INTEREST
I.J Núñez-Gil is a consultant for Aztraseneca. P. Salinas received speaker fees from Boston Scientific, Terumo, Alvinedica, and Biomenco. L. Nombela-Franco has served as a proctor for Abbott, and received speaker fees from Edwards Lifesciences Inc. A. Fernández-Ortiz is a speaker at the educational events of Medtronic, Biotronik, Biosensor, and Bayer. J. Escaned is a speaker and consultant for Abbott, Boston Scientific, and Philips, and received personal fees from Philips Volcano, Boston Scientific, and Abbott/St. Jude Medical outside the submitted work. N. Gonzalo is a speaker at educational events for Abbott, and Boston Scientific. The remaining authors declared no conflicts of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
- The FFR and the iFR have proven to be safe tools to guide revascularization treatment in several clinical scenarios at the long-term follow-up. However, the safety of physiology-based revascularization in diabetics, who have a high-risk of cardiovascular events, has been scarcely investigated.
WHAT DOES THIS STUDY ADD?
- Deferring the revascularization of intermediate stenosis in diabetic patients based on the results of physiological evaluation with pressure guidewires is safe, and has a low rate of secondary events being the deferred vessel similar to those seen in non-diabetic patients at the longterm follow-up.
REFERENCES
1. Neumann F-J, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2018;40:87-165.
2. Pijls NH, Fearon WF, Tonino PA, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease:2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56:177-184.
3. Davies JE, Sen S, Dehbi HM, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. New Engl J Med. 2017;376:1824-1834.
4. Escaned J, Ryan N, Mejia-Renteria H, et al. Safety of the Deferral of Coronary Revascularization on the Basis of Instantaneous Wave-Free Ratio and Fractional Flow Reserve Measurements in Stable Coronary Artery Disease and Acute Coronary Syndromes. JACC Cardiovasc Interv. 2018;11:1437-1449.
5. Zimmermann FM, Ferrara A, Johnson NP, et al. Deferral vs performance of percutaneous coronary intervention of functionally non-significant coronary stenosis:15-year follow-up of the DEFER trial. Eur Heart J. 2015;36:3182-3188.
6. Gotberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. New Engl J Med. 2017;376:1813-1823.
7. Escaned J, Collet C, Ryan N, et al. Clinical outcomes of state-of-the-art percutaneous coronary revascularization in patients with de novo three vessel disease:1-year results of the SYNTAX II study. Eur Heart J. 2017;38:3124-3134.
8. van Nunen LX, Zimmermann FM, Tonino PA, et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME):5-year follow-up of a randomised controlled trial. Lancet. 2015;386:853-1860.
9. Norhammar A, Malmberg K, Diderholm E, et al. Diabetes mellitus:the major risk factor in unstable coronary artery disease even after consideration of the extent of coronary artery disease and benefits of revascularization. J Am Coll Cardiol. 2004;43:585-591.
10. Castro Mejia A, Ortega Armas M, Lopez Ferreo L. Factores de riesgo en pacientes con cardiopatía isquémica angiográficamente severa:diferencias según sexo. Rev Cuba Cardiol Cir Cardiovasc. 2015;21:7p.
11. Esper RB, Farkouh ME, Ribeiro EE, et al. SYNTAX Score in Patients With Diabetes Undergoing Coronary Revascularization in the FREEDOM Trial. J Am Coll Cardiol. 2018;72:2826-2837.
12. Jeremias A, Kirtane AJ, Stone GW. A Test in Context. Fractional Flow Reserve:Accuracy, Prognostic Implications, and Limitations. J Am Coll Cardiol. 2017;69:2748-2758.
13. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. New Engl J Med. 2009;360:213-224.
14. Kim U, Leipsic JA, Sellers SL, et al. Natural History of Diabetic Coronary Atherosclerosis by Quantitative Measurement of Serial Coronary Computed Tomographic Angiography. Results of the PARADIGM Study (Progression of Atherosclerotic Plaque Determined by Computed Tomographic Angiography Imaging). J Am Coll Cardiol Img. 2018:11:1461-1471.
15. Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of Diabetes on Progression of Coronary Atherosclerosis and Arterial Remodeling:A Pooled Analysis of 5 Intravascular Ultrasound Trials. J Am Coll Cardiol.2008;52:255-262.
16. Xaplanteris P, Fournier S, Pijls NHJ, et al. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. New Engl J Med. 2018;379:250-259.
17. De Bruyne B, Fearon WF, Pijls NH, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. New Engl J Med. 2014;371:1208-1217.
18. Domínguez-Franco AJ, Jiménez-Navarro MF, Muñoz-García AJ, et al. Pronóstico a largo plazo de diferir la intervención coronaria en diabéticos sobre la base de la reserva fraccional de flujo. Rev Esp Cardiol. 2008;61:352-359.
19. Kennedy MW, Kaplan E, Hermanides RS, et al. Clinical outcomes of deferred revascularisation using fractional flow reserve in patients with and without diabetes mellitus. Cardiovasc Diabetol. 2016;15:100.
20. Kennedy MW, Fabris E, Hermanides RS, et al. Factors associated with deferred lesion failure following fractional flow reserve assessment in patients with diabetes mellitus. Catheter Cardiovasc Interv.2017;90:1077-1083.
21. Van Belle E, Cosenza A, Baptista SB, et al. Usefulness of Routine Fractional Flow Reserve for Clinical Management of Coronary Artery Disease in Patients With Diabetes. JAMA Cardiol. 2020;5:272-281.
22. Lee JM, Choi KH, Koo BK, et al. Comparison of Major Adverse Cardiac Events Between Instantaneous Wave-Free Ratio and Fractional Flow Reserve-Guided Strategy in Patients With or Without Type 2 Diabetes:A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2019;4:857-864.
23. Petraco R, van de Hoef TP, Nijjer S, et al. Baseline instantaneous wave-free ratio as a pressure-only estimation of underlying coronary flow reserve:results of the JUSTIFY-CFR Study (Joined Coronary Pressure and Flow Analysis to Determine Diagnostic Characteristics of Basal and Hyperemic Indices of Functional Lesion Severity-Coronary Flow Reserve). Circ Cardiovasc Interv. 2014;7:492-502.
24. Cook CM, Jeremias A, Petraco R, et al. Fractional Flow Reserve/Instantaneous Wave-Free Ratio Discordance in Angiographically Intermediate Coronary Stenoses:An Analysis Using Doppler-Derived Coronary Flow Measurements. JACC Cardiovasc Interv. 2017;10:2514-2524.
* Corresponding author: Departamento de Cardiología Intervencionista, Instituto Cardiovascular, Hospital Universitario Clínico San Carlos, IdISSC, Universidad Complutense, Prof. Martín Lagos s/n, 28040 Madrid, Spain.
E-mail address: nieves_gonzalo@yahoo.es (N. Gonzalo).











