ABSTRACT
Introduction and objectives: Distal embolization and no-reflow are common complications in primary angioplasty and the information available on the role played by the deflation speed of the stent delivery system is scarce. Our aim is to analyze how the deflation speed of the stent delivery system impacts the results of primary angioplasty.
Methods: From December 2016 through February 2019, all consecutive patients with ST-segment elevation myocardial infarction undergoing urgent coronary angiography at our institution and who were eligible for thrombectomy, IIB-IIIA inhibitors, and direct stenting were randomized in a 1:1 ratio to rapid (group 1, n = 103) or slow deflation of the stent delivery system, at 1 atm/second, (group 2, n = 107). Pre- and postdilatation was not allowed per protocol. The primary outcomes were myocardial blush ≥ 2 and ST-segment resolution ≥ 70% while the size of myocardial damage, the ejection fraction both at discharge and at the 12-month follow-up, and the overall and 12-month cardiovascular mortality rates were the secondary outcomes.
Results: The study was stopped prematurely with 50% of the estimated sample size due to futility. Myocardial blush ≥ 2 occurred in 77 (74.7%) vs 79 (75.2%) of the patients, P = .93, and ST-segment resolution ≥ 70% occurred in 54 (53.9%) vs 59 (55.5%) of the patients, P = .75 in groups 1 and 2, respectively without any differences being reported in any of the secondary endpoints.
Conclusions: In our series, the deflation speed of the stent delivery system in primary angioplasty did not modify the myocardial blush ≥ 2, the ST-segment resolution ≥ 70% or impacted the clinical outcomes, the size of myocardial infarction according to the biomarkers or the ejection fraction.
Keywords: Primary angioplasty. ST-segment-elevation myocardial infarction. No-reflow. ST-segment resolution. Myocardial blush.
RESUMEN
Introducción y objetivos: La embolización distal y el fenómeno de no-reflow son complicaciones frecuentes de la angioplastia primaria. La información disponible sobre la influencia de la velocidad de desinflado del sistema de liberación del stent es escasa. Nuestro objetivo es analizar la influencia de este factor en los resultados de la angioplastia primaria.
Métodos: Entre diciembre de 2016 y febrero de 2019, todos los pacientes consecutivos con infarto de miocardio con elevación del segmento ST sometidos a coronariografía urgente en nuestro centro y que eran susceptibles de trombectomía, inhibidores de IIB-IIIA e implante directo de stent fueron aleatorizados 1:1 a un desinflado rápido del sistema de liberación (grupo 1, n = 103) o a un desinflado lento a 1 atm/s (grupo 2, n = 107). Por protocolo, no se permitió la predilatación previa ni posterior. Los objetivos primarios fueron el grado de blush miocárdico ≥ 2 y la resolución del segmento ST ≥ 70%. Los objetivos secundarios fueron el tamaño del infarto, la fracción de eyección al alta y a los 12 meses, y las mortalidades total y cardiovascular a los 12 meses.
Resultados: El estudio se detuvo prematuramente con el 50% del tamaño muestral calculado por futilidad. Se encontró blush ≥ 2 en 77 (74,7%) frente a 79 (75,2%) pacientes (p = 0,93) y resolución del segmento ST ≥ 70% en 54 (53,9%) frente a 59 (55,5%) pacientes (p = 0,75) en los grupos 1 y 2, respectivamente, sin diferencias en ninguno de los objetivos secundarios.
Conclusiones: En nuestra serie, la velocidad de desinflado del sistema de liberación del stent en la angioplastia primaria no modificó el blush miocárdico ni la resolución del segmento ST, y tampoco demostró tener influencia en los resultados clínicos, el tamaño del infarto según los biomarcadores ni la fracción de eyección.
Palabras clave: Angioplastia primaria. Infarto con elevacion del segmento ST. No-reflow. Resolucion del segmento ST. Blush miocardico.
Abbreviations MB: myocardial blush. pPCI: primary percutaneous coronary intervention. STR: ST-segment resolution. STEMI: ST-segment elevation myocardial infarction. TIMI: Thrombolysis in Myocardial Infarction.
INTRODUCTION
Distal embolization and slow coronary flow often limit the success of primary percutaneous coronary angioplasty (pPCI). In 25% to 50% of the cases, despite satisfactory flow restoration, poor microvascular reperfusion can be seen, which leads to worse prognoses.1 This is a field of ongoing discussion because strategies that initially showed positive results have later been questioned like direct stenting,2 thrombus aspiration,3,4 and the administration of beta-blockers,5 and IIB-IIIA inhibitors.6
It has been confirmed that aggressive balloon dilatation with a high balloon-to-artery ratio may favor the presence of no-reflow and it has been speculated that the deflation speed of the stent delivery system may impact the results too, although the information available on this regard is scarce.
No-reflow may be due to different pathophysiological factors such as distal embolization, ischemia-reperfusion injury, and the susceptibility of coronary microcirculation to injury.7,8 Rapid stent balloon deflation may trigger the so-called siphon effect and rapid changes in coronary hemodynamics that can be associated with distal embolization, and microcirculatory dysfunction.9 As part of a published report, the investigators built an in vitro experimental study and combined it with a computer model to eventually find that the wall shear stress due to the different balloon deflation strategies used triggered differences in the flow final velocity as well.
Our objective is to analyze the impact of the deflation speed of the stent delivery system on myocardial blush (MB), and the ST-segment resolution (STR) in the acute phase, as well as the prognosis and ejection fraction at the 12-month follow-up.
METHODS
Patients
A randomized, parallel, single-center study was conducted with a 24-hour program of pPCI including 440 000 patients. Recruitment was carried out by convenience sampling and eligible patients were all consecutive subjects with ST-segment elevation myocardial infarction (STEMI) referred to receive a pPCI who had a culprit lesion eligible for direct stenting. Patients should have ST-segment elevations ≥ 0.1 mV in 2 contiguous leads or new left bundle branch block.
Exclusion criteria were contraindications to acetylsalicylic acid, clopidogrel or IIB-IIIA inhibitors, impossibility to complete the follow-up, life expectancy < 12 months, lesion not amenable to direct stenting, culprit lesions located at grafts or in-stent thrombosis, and previous oral anticoagulation.
After performing the coronary angiography, the patients who met the inclusion criteria and had no exclusion criteria gave their initial oral consent and were allocated by simple randomization through a computer-generated list that would create individual codes. These codes were inserted one by one in identical envelopes—prepared by personnel not involved in the study—that were thick enough so the codes could not be seen. All patients were asked to confirm their participation by giving their written informed consent within 24 hours. The study protocol was designed in full compliance with the ethical guidelines of the 1975 Declaration of Helsinki as shown in a prior approval granted by the center human research committee.
Parallel groups were created by a) direct stenting with fast deflation of the stent delivery system after 20 seconds of balloon inflation (group 1), or b) direct stenting with slow deflation at 1 atm/second after the same period of inflation (group 2).
Procedure
Patients and outcome evaluators were blind to the procedure. To minimize variability and any potential confounders the protocol was strict and included the administration of 250 mg of acetylsalicylic acid followed by 600 mg of clopidogrel at the first medical contact (according to the myocardial infarction protocol of our unit), 70 mg/kg of IV heparin, and IV abciximab or tirofiban at the beginning of the procedure for a 12-hour administration course. Manual thrombectomy and posdilatation of the stent were performed systematically, but implantation of a second stent was not allowed per protocol. Intention-to-treat and per protocol analyses were performed. The former dictated the main analysis. The volume of contrast per injection was 6 mL administered for 3 seconds into the left main coronary artery followed by 4 mL administered for 2 seconds into the right coronary artery using the ACIST device (ACIST Medical Systems Inc., United States). Intracoronary nitroglycerine (100 µg to 200 µg) was administered before the final injection to assess MB. Myocardial blush was studied in the right anterior oblique 20-degree projection with 20-degree caudal angulation, and in the left anterior oblique 45-degree projection with 20-degree cranial angulation regarding the left main coronary artery, and in the anteroposterior projection with 20-degree cranial angulation regarding the right coronary artery. Recordings were acquired at 30 images/second without image magnification with a prolonged duration until the venous phase of the myocardial circulation was completed.
Within the first 30 minutes upon arrival to the coronary care unit, patients underwent a 12-lead electrocardiogram and blood samples were obtained for troponin I assessment 6 and 24 hours after the procedure, as well as additional measurements until a reduction in the levels reported was confirmed.
Optimal medical management according to guidelines was recommended with statins, beta-blockers, or renin-angiotensin system blockers. Also, dual antiplatelet therapy was indicated for 12 months. Switching to ticagrelor during admission was also recommended in the absence of significant risk of bleeding.
Outcomes
The 2 primary endpoints were how the deflation speed of the stent delivery system impacted MB at the end of the procedure, and the STR. The final MB was analyzed blindly by an external core laboratory in a different region and the variable analyzed was the percentage of MB grade ≥ 2 vs < 2 between both groups by visual assessment. Two interventional cardiologists with > 10 years of experience grading MBs10 were involved in the evaluation and, in case of disagreement, a third opinion was requested. The STR was analyzed by evaluators not involved in the study who were blind to the procedure. The J-point was manually identified with respect to the nearest 0.5 mm in all leads except in the aVR lead. Using the TP segment as the isoelectric baseline interval, the extent of the ST-segment elevation with respect to the nearest 0.05 mV was measured 80 ms after the J-point. The STR was estimated by a reduction in the sum of the ST-segment elevation in all leads except in the aVR from the baseline ECG compared to the ECG performed upon arrival at the coronary care unit. The variable was a binary outcome, the ≥ 70% resolution of the sum of millimeters of ST-elevation between both recordings.
The secondary endpoints were: a) size of the myocardial damage comparing the maximum levels of troponin I; b) ejection fraction at discharge; c) ejection fraction at 12 months; d) all-cause mortality rate at 12 months; and e) 12-month cardiovascular mortality rate.
Definitions
Angiographic thrombus burden was defined according to Sianos’ classification11 while collateral supply was defined according to Rentrop classification.12
Quantitative coronary angiography
The Medis Suite XA system (Medis Medical Imaging, Israel) was used for the analysis according to the experts’ standards.13 Lesion length was measured once the vessel flow had been restored after thrombectomy. The diameter parameters were taken at the end of the procedure after the stent was deployed due to the difficulties reported while performing analyses in thrombotic vessels. The following data were used: reference vessel diameter (the average lumen diameter assumed without atherosclerotic disease), minimal lumen diameter, postoperative stenosis, and the stent-to-artery ratio.
Sample size calculations
Based on a primary endpoint of STR of 50% in the control group14,15 and an increase up to 62.5% in the procedural group following, the principle of minimum clinically significant difference between treatments of 25%,16 and a dropout rate of 10%, 420 patients, 210 per group, were needed.
Interim analysis
Given the uncertainty of the results and the lack of data available on the medical literature, an interim futility analysis was planned after recruiting 50% of the sample size.
Statistical analysis
Quantitative variables with normal distribution were expressed as means and standard deviation, and those without a normal distribution as median and interquartile range. Categorical variables were expressed as absolute values and percentages. The mean comparison was carried out using the Student t test in normal distribution or the Mann-Whitney U test when that assumption was not met. The chi-square test or Fisher’s exact test were used to compare proportions. Two-tailed tests were used to analyze all studies. P values ≤ .05 were considered statistically significant. A logistic regression analysis was performed to adjust for possible imbalances and measure how the deflation speed rate of the stent delivery system impacted each of the 2 primary endpoints. The variables that met the 2 criteria of a reasonable association with the outcomes and P values < .20 in the univariate analysis were tested in the multivariate analysis. The calculations were performed using the SPSS 27.0.0.0 statistical software (IBM Corp, United States).
RESULTS
Baseline
From December 2016 through February 2019 a total of 447 patients were referred to our cath lab with a diagnosis of STEMI (figure 1, flow diagram). A total of 237 (53%) were not eligible for randomization and the remaining 210 (47%) were allocated to fast (103, 49%) or slow balloon deflation (107, 51%). The initially calculated sample size was 420 patients but, after an interim analysis with 50% of the sample recruited, the study was terminated early due to futility. There was 1 protocol violation in the first group and 4 in the second group. The intention-to-treat analysis is seen in this section and the per protocol analysis on tables 3 to 5 of the supplementary data. The baseline and procedural characteristics of the study cohort are shown on table 1 and table 2. There were no statistical differences between both groups although, despite the randomization process, there was a non-significant trend towards a larger vessel diameter in the slow deflation group. All cases were performed with 6-Fr guiding catheters.
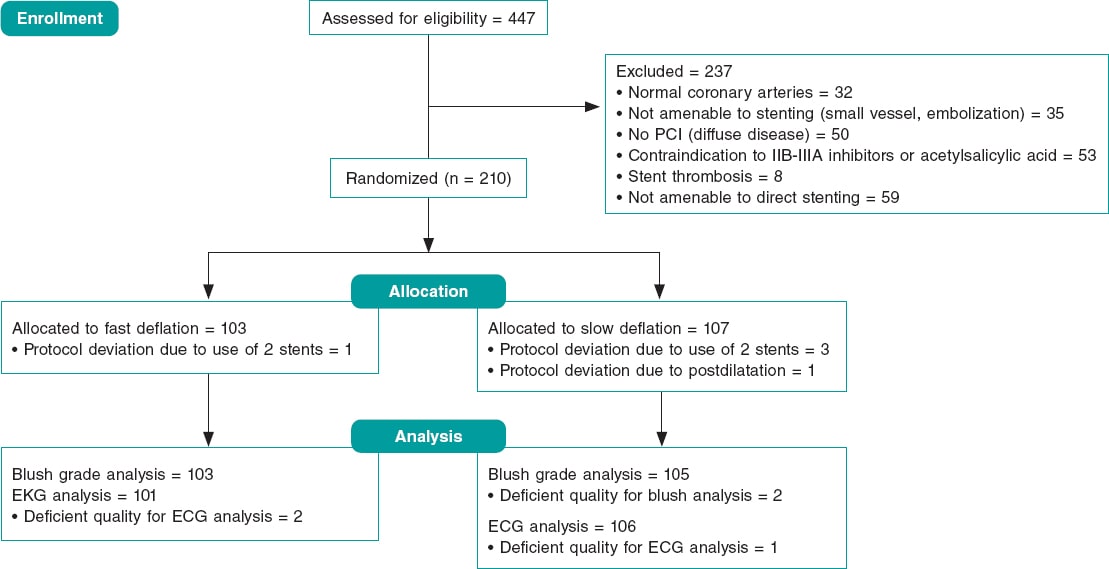
Figure 1. Study flow-chart. ECG, electrocardiogram; PCI, percutaneous coronary intervention.
Table 1. Baseline clinical characteristics
| Fast deflation N = 103 | Slow deflation N = 107 | P | |
|---|---|---|---|
| Age | 59.73 (10.56) | 59.33 (10.71) | .78 |
| Sex (female) | 26 (25.2) | 20 (18.7) | .25 |
| Diabetes | 14 (13.6) | 21 (19.6) | .24 |
| Hypertension | 40 (38.9) | 48 (44.8) | .37 |
| Hypercholesterolemia | 37 (35.9) | 45 (42.1) | .36 |
| Smoking | 65 (63.1) | 71 (66.3) | .62 |
| Previous myocardial infarction | 4 (3.9) | 6 (5.6) | .75 |
| Previous percutaneous coronary intervention | 3 (2.9) | 4 (3.7) | 1.00 |
| Previous coronary artery bypass graft | 0 (0) | 1 (0.1) | 1.00 |
| Previous stroke | 1 (0.1) | 0 (0.0) | .49 |
| Creatinine clearance levels < 60 mL/min | 14 (13.6) | 22 (20.5) | .18 |
| Blood pressure at admission | 123.4 (30.8) | 129.6 (28) | .13 |
| Shock | 4 (3.9) | 1 (0.09) | .21 |
| Radial access | 103 (100) | 105 (98.1) | .50 |
| Number of diseased vessels | 1.38 (0.61) | 1.45(0.66) | .42 |
| Total ischemic time | 192 (125-295) | 169 (120-260) | .21 |
| First medical visit to balloon time | 87 (66-130) | 80 (65-114) | .22 |
| ST elevation before procedure (mm) | 11.40 (6.74) | 12.63 (8.06) | .24 |
Quantitative variables with normal distribution are expressed as means and standard deviation (SD), variables with non-normal distribution as median and interquartile range, and categorical variables are expressed as absolute values and percentages. | |||
Table 2. Characteristics of the procedure
| Fast deflation N = 103 | Slow deflation N = 107 | P | |
|---|---|---|---|
| Vessel | .60 | ||
| Left anterior descending coronary artert | 44 (42.7) | 40 (37.4) | |
| Left circumflex artery | 13 (12.6) | 18 (16.8) | |
| Right coronary artery | 46 (44.7) | 49 (45.8) | |
| Preoperative TIMI ≥ grade 2 flowa | 10 (9.7) | 17 (15.9) | .21 |
| Rentrop ≥ 2 | 15 (14.6) | 19 (17.8) | .53 |
| Thrombus grade score ≥ 4 | 46 (44.6) | 50 (46.7) | .76 |
| Drug-eluting stent | 100 (97.1) | 101 (94.4) | .50 |
| Percent diameter stenosis | 99.28 (3.43) | 98.89 (6.48) | .58 |
| RVDb | 2.74 (0.42) | 2.86 (0.47) | .07 |
| Lesion length | 14.07 (5.94) | 13.44 (4.71) | .39 |
| Stent diameter | 3.23 (0.47) | 3.32 (0.57) | .17 |
| Maximum inflation pressure | 14.68 (1.48) | 14.77 (1.69) | .67 |
| MLDc | 2.89 (0.38) | 3.00 (0.49) | .06 |
| Minimum lumen diameter | 2.63 (0.39) | 2.67 (0.48) | .48 |
| Postoperative stenosis | 8.92 (4.75) | 11.20 (6.25) | .01 |
| Stent-to-artery ratio | 1.05 (0.07) | 1.05 (0.08) | .95 |
Quantitative variables with normal distribution are expressed as means and standard deviation (SD), variables with non-normal distribution as median and interquartile range, and categorical variables are expressed as absolute values and percentages. a TIMI, Thrombolysis in Myocardial Infarction risk score. b RVD, reference vessel diameter after the procedure. c MLD, maximum lumen diameter after the procedure. | |||
Table 3. Results
| Fast deflation N = 103 | Slow deflation N = 107 | P | |
|---|---|---|---|
| Myocardial blush ≥ 2 | 77 (74.7) | 79 (75.2) | .93 |
| Postoperative ST-segment elevation (mm) | 4.26 (5.19) | 4.03 (4.69) | .73 |
| ST-segment elevation resolution (mm) | 7.03 (6.99) | 8.56 (8.11) | .15 |
| Percentage of resolution (%) | 64.97 (33.35) | 65.40 (34.69) | .92 |
| Resolution ≥ 70 % | 54 (53.4) | 59 (55.6) | .75 |
| TIMI grade flow after the procedure | .38 | ||
| 0 | 1 | 0 | |
| 1 | 0 | 1 | |
| 2 | 5 | 9 | |
| 3 | 97 | 97 | |
| Maximum troponin-I levels | 47.84 (14-129) | 72 (29.7-144.75) | .14 |
| Ejection fraction at discharge | 53.9 (8.58) | 54.62 (8.71) | .55 |
| Ejection fraction at 12 months | 57.43 (8.20) | 57.75 (6.48) | .76 |
| In-hospital mortality rate | 1 (0.9) | 2 (1.8) | 1.00 |
| Overall mortality rate at 12 months | 3 (2.9) | 3 (2.8) | 1.00 |
| Cardiovascular mortality rate at 12 months | 2 (1.9) | 3 (2.8) | 1.00 |
| Myocardial infarction | 1 (0.9) | 1 (0.9) | 1.00 |
| Target vessel revascularization | 0 | 1 (0.9) | 1.00 |
Quantitative variables with normal distribution are expressed as means and standard deviation (SD), variables with non-normal distribution as median and interquartile range, and categorical variables are expressed as absolute values and percentages. TIMI, Thrombolysis in Myocardial Infarction risk score. | |||
Table 4. Predictors of myocardial blush ≥ 2 and ST-segment resolution
| OR | 95%CI | P | |
|---|---|---|---|
| Predictors of myocardial blush ≥ 2 | |||
| Systolic blood pressure at admission | 1.02 | 1.02-1.03 | .011 |
| Creatinine clearance levels <60 mL/min | 0.29 | 0.13-0.66 | .003 |
| Postoperative maximum lumen diameter | 3.08 | 1.24-7.63 | .015 |
| Hypertension | 0.52 | 0.26-1.06 | .074 |
| Predictors of ST-segment resolution | |||
| Diabetes | 0.16 | 0.06-0.43 | < .001 |
| Previous myocardial infarction | 13.54 | 1.47-124.91 | .022 |
| Left anterior descending coronary artery | 0.46 | 0.24-0.91 | .025 |
| Preoperative TIMI grade flow ≥ 2 | 3.95 | 1.36-11.46 | .011 |
| Postoperative TIMI grade 3 flow | 7.10 | 1.76-28.68 | .006 |
| Rentrop grade ≥ 2 collateral circulation | 0.31 | 0.13-0.75 | .010 |
Quantitative variables with normal distribution are expressed as means and standard deviation (SD), variables with non-normal distribution as median and interquartile range, and categorical variables are expressed as absolute values and percentages. 95%CI, 95% confidence interval; OR, odds ratio; TIMI, Thrombolysis in Myocardial Infarction risk score. | |||
Endpoints
The primary endpoint, MB grade ≥ 2compared to < 2, occurred in 77 (74.7%) vs 79 (75.2%), P = .93, of the patients, and STR ≥ 70% in 54 (53.9%) vs 59 (55.5%), P = .75, of the patients from the rapid and slow deflation groups, respectively. Also, there were no differences in any of the secondary endpoints regarding the size of myocardial damage, the ejection fraction at discharge, the ejection fraction at 12 months, the overall mortality rate at 12 months or in the cardiovascular mortality rate at 12 months (table 3).
Predictors of myocardial blush
The univariate analysis was performed with the variables shown on table 1 of the supplementary data. The variables age, creatinine clearance levels < 60 mL/min, postoperative maximum lumen diameter, past medical history of hypertension, systolic blood pressure at admission, Rentrop grade ≥ 2 collateral circulation, and the first medical contact to balloon time were tested using a logistic regression model. Systolic blood pressure at admission, creatinine clearance levels < 60 mL/min, and the postoperative maximum lumen diameter were predictors of blush ≥ 2 while in the final model hypertension remained with P values = .074 (table 4). The predictive power was moderate with an area under the ROC curve of 0.71 (0.63-0.80) (figure 2).
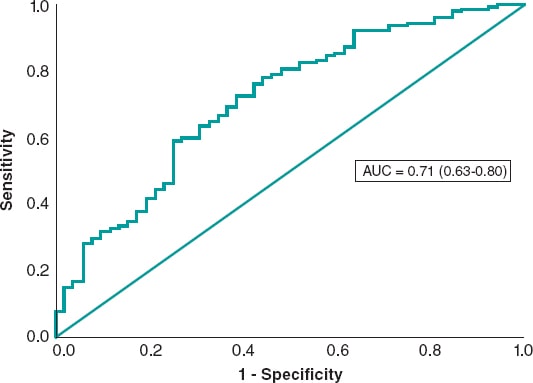
Figure 2. Receiver operating characteristic curve of the logistic regression model for myocardial blush prediction.
Predictors of ST-segment resolution ≥ 70%
The univariate analysis was performed with the variables listed on table 2 of the supplementary data. The variables tested in the multivariate analysis were sex, diabetes, hypercholesterolemia, smoking, shock, left anterior descending coronary artery, previous myocardial infarction, preoperative TIMI grade ≥ 2 flow, postoperative TIMI grade 3 flow, and Rentrop grade ≥ 2 collateral circulation, number of millimeters of ST elevation before the procedure, and creatinine clearance levels < 60 mL/min. The logistic regression model included diabetes, previous myocardial infarction, left anterior descending coronary artery, preoperative TIMI grade ≥ 2 flow, postoperative TIMI grade 3 flow, and Rentrop grade ≥ 2 collateral circulation as predictors of ST-segment resolution ≥ 70% (table 4). The area under the ROC curve was 0.75 (0.68-0.82) (figure 3).
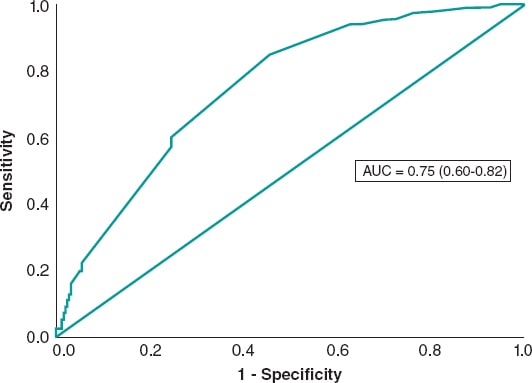
Figure 3. Receiver operating characteristic curve of the logistic regression model for ST-segment resolution.
Per protocol analysis
Protocol deviation was seen in 5 patients. In the rapid deflation group 2 stents were needed in 1 patient. In the slow deflation group 3 patients received 2 stents followed by 1 postdilatation (figure 1). Tables 3, 4 and 5 of the supplementary data show the per protocol analysis without any significant differences compared to the intention-to-treat analysis.
Missing values
In 2 patients from the slow deflation group, the quality of the angiogram did not allow us to perform a proper analysis. Regarding the electrocardiogram, suboptimal quality was recorded in 2 patients from the rapid deflation group and in 1 patient from the slow deflation group. All of them may be considered as missing values completely at random, which means that the randomization balance was never affected.
DISCUSSION
In this randomized study we assessed how the deflation speed of the stent delivery system impacted myocardial blush ≥ 2, and ST-segment resolution ≥ 70%. The most important findings are: a) the study was stopped with 50% of the predefined sample sized due to futility and neither MB nor STR were modified by the intervention; b) no differences were seen in the size of myocardial damage, ejection fraction at 12 months and discharge or in the all-cause and 12-month cardiovascular mortality rates; c) systolic blood pressure at admission, creatinine clearance levels < 60 mL/min, and postoperative maximum lumen diameter played a role in MB while the past medical history of hypertension would have probably been included in the final model if the sample size would have been larger; and d) STR was influenced by diabetes, previous myocardial infarction, left anterior descending coronary artery, preoperative TIMI grade ≥ 2 flow, postoperative TIMI grade 3 flow, and Rentrop grade ≥ 2 collateral circulation.
The data available on the medical literature on this research topic is significantly scarce and, to our knowledge, only 1 group has provided information. Gu et al.17 also studied the association of balloon deflation during stent deployment with coronary flow and clinical outcomes regarding pPCI in a series of 211 patients. They found that slow deflation led to favorable coronary flow and infarct size compared to conventional rapid deflation. These contradictory results may be justified by the remarkable differences seen between both cohorts. Former studies have reported on the role of balloon inflation,18 thrombectomy,19,20 and IIB-IIIA inhibition21 in the management of MB. In our series, we designed a strict protocol to control these potential confounders, which is why pre- and postdilatation was not allowed, and both thrombus aspiration and IIB-IIIA inhibitors were essential components of the procedure. The study conducted by Gu et al. allowed both pre- and postdilatation while the use of thrombectomy, and IIB-IIIA inhibitors was left to the operator’s discretion. Indeed, predilatation was performed in > 80% of the patients from both groups, postdilatation in roughly 40%, thrombus aspiration in only 20%, and IIB-IIIA inhibitors were administered in 70% of the patients. Undoubtedly, the approach conducted by Gu et al. favored external validity although, in our opinion, the influence of these 4 factors may have influenced the results deeply, mainly when no adjustment was performed through a multivariate analysis. Finally, although closely related, the TIMI frame count and MB are not the same endpoint, and the ST-segment resolution was not assessed in the study conducted by Gu et al. Regarding the clinical endpoints, no differences were seen between the 2 strategies in any of the 2 studies.
As we mentioned, we were not able to show that the deflation speed of the stent delivery system impacted MB. In the multivariate analysis performed, blood pressure levels at admission, creatinine clearance levels, and the postoperative maximum lumen diameter were all predictors of MB while a past medical history of hypertension would have probably reached statistical significance with a larger sample size. Former reports have underlined how blood pressure impacts MB during the procedure.22 Also, patients with hypertension due to an increased microvascular resistance have shown an impaired flow.22 In addition, it has been reported that the adverse event of renal function regarding cardiovascular events may be mediated by an increased microvascular resistance.23 Time to treatment has impacted MB in previous studies.24 In our cohort, there were significant differences in the univariate analysis, but in the last step of the multivariate analysis it was removed from the final model, although it would have probably been present with a larger sample size. However, in the comparison of our series with the aforementioned study, we tested the vessel size as a predictor of MB while this variable was not analyzed in Luca’s study, but it had played a role in previous cohorts.25
Consistent with this, the deflation speed of the stent delivery system did not seem to play a role in STR. We found up to 6 factors that proved its impact on the ST-segment resolution, most of them already described in former studies. As it leads to a lower ST-segment elevation, collateral circulation reduces the impact of pPCI in STR.26 Anterior infarctions with culprit lesion in the left anterior descending coronary artery also led to lower ST-segment recoveryies in previous cohorts.27-29 This was also seen with preoperative TIMI grade < 2 flow, and final TIMI grade flow < 3,14,27,28,30 and diabetes.14,28 In our series, previous myocardial infarction was a predictor of STR, although we found no explanation for this finding.
Limitations
The study was stopped in the interim analysis based on the criterion of futility. However, we do not expect the results of primary endpoints to have been any different with the whole sample size. We could have probably found more predictors and a higher predictive power of the MB and STR models, but this was not the endpoint of our study. The risk profile of the patients was low because the inclusion criteria of direct stenting, use of IIB-IIIA inhibitors, and thrombectomy focused the study on lesions more frequently associated with younger patients with a low bleeding risk and less calcification, which are features associated with better outcomes. This limits the external validity of the study because, as shown on figure 1, roughly 50% of the patients were ineligible to enter the study. This may have also played a role in the lack of differences seen between the study groups. However, as we have already explained, the purpose of our study was to avoid any confounders. Clopidogrel was the P2Y12 inhibitor at the first medical visit according to the protocol of the regional myocardial infarction network of our area. This may also limit the external validity of the results. Myocardial blush was visually assessed and, although it was performed by 2 experienced operators, certain degree of subjectivity cannot be ruled out. The predictive power for both MB and STR was low, but it has also occurred in former series28 being the concordance between those factors described as moderate.31 Finally, we could not find any explanations for the role of previous myocardial infarction predicting STR as this factor was not present in former series.
CONCLUSIONS
In our series, the deflation speed of the stent delivery system in primary angioplasty did not change myocardial blush or ST-segment resolution and no influence was seen on the clinical outcomes, size of myocardial infarction assessed by biomarkers, and ejection fraction at discharge and after 12 months.
FUNDING
The study has been supported by a research grant from Abbott Laboratories.
AUTHORS’ CONTRIBUTIONS
B. Vega, J. M. Vegas, J. Rondan, E. Segovia, and Í. Lozano: design, data mining, manuscript drafting, and manuscript revision. A. Pérez de Prado, C. Cuellas-Ramon, M. López-Benito, T. Benito-González, and F. Hernández-Vázquez: blush measurements, and manuscript revision.
CONFLICTS OF INTEREST
The authors declared no conflict of interests whatsoever.
WHAT IS KNOWN ABOUT THE TOPIC?
- Distal embolization and slow coronary flow frequently reduce the success of primary angioplasty.
- Several interventions have been tested but it is a field of ongoing debate because the strategies that showed positive results at the beginning have now been questioned such as direct stenting, thrombus aspiration, and use of beta-blockers and IIB-IIIA inhibitors.
- It has been demonstrated that aggressive balloon dilatation with a high balloon to artery ratio may favor the presence of no-reflow. Also, it has been speculated that the deflation speed of the stent delivery system may impact the results, although the information available on this regard is scarce.
WHAT DOES THIS STUDY ADD?
- Our objective is to analyze how the deflation speed of the stent delivery system impacts myocardial blush, ST-segment resolution in the acute phase, prognosis, and the ejection fraction at 12months.
- The study was prematurely stopped due to futility because the speed of deflation of the stent delivery system did not change the primary outcomes or impacted the size of the infarction, prognosis or the ejection fraction at 12 months whatsoever.
REFERENCES
1. Ito H, Tomooka T, Sakai N, et al. Lack of myocardial perfusion immediately after successful thrombolysis. A predictor of poor recovery of left ventricular function in anterior myocardial infarction. Circulation. 1992;85:1699-1705.
2. Mahmoud KD, Jolly SS, James S, et al. Clinical impact of direct stenting and interaction with thrombus aspiration in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention:Thrombectomy Trialists Collaboration. Eur Heart J. 2018;39:2472-2479.
3. Frobert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587-1597.
4. Jolly SS, Cairns JA, Yusuf S, et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction:1-year follow-up of the prospective randomised TOTAL trial. Lancet. 2016;387:127-135.
5. Roolvink V, Ibanez B, Ottervanger JP, et al. Early Intravenous Beta-Blockers in Patients With ST-Segment Elevation Myocardial Infarction Before Primary Percutaneous Coronary Intervention. J Am Coll Cardiol. 2016;67:2705-2715.
6. Ellis SG, Tendera M, de Belder MA, et al. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med. 2008;358:2205-2217.
7. Konijnenberg LSF, Damman P, Duncker DJ, et al. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc Res. 2020;116:787-805.
8. Dong M, Mu N, Guo F, et al. The beneficial effects of postconditioning on no-reflow phenomenon after percutaneous coronary intervention in patients with ST-elevation acute myocardial infarction. J Thromb Thrombolysis. 2014;38:208-214.
9. Li R, Zijlstra JG, Kamps JA, van Meurs M, Molema G. Abrupt reflow enhances cytokine-induced proinflammatory activation of endothelial cells during simulated shock and resuscitation. Shock. 2014;42:356-364.
10. Perez de Prado A, Fernandez-Vazquez F, Cuellas-Ramon JC, Iglesias-Garriz I. Coronary clearance frame count:a new index of microvascular perfusion. J Thromb Thrombolysis. 2005;19:97-100.
11. Sianos G, Papafaklis MI, Daemen J, et al. Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction:the importance of thrombus burden. J Am Coll Cardiol. 2007;50:573-583.
12. Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587-592.
13. Suzuki N, Asano T, Nakazawa G, et al. Clinical expert consensus document on quantitative coronary angiography from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc Interv Ther. 2020;35:105-116.
14. Farkouh ME, Reiffel J, Dressler O, et al. Relationship between ST-segment recovery and clinical outcomes after primary percutaneous coronary intervention:the HORIZONS-AMI ECG substudy report. Circ Cardiovasc Interv. 2013;6:216-223.
15. Fabris E, van 't Hof A, Hamm CW, et al. Clinical impact and predictors of complete ST segment resolution after primary percutaneous coronary intervention:A subanalysis of the ATLANTIC Trial. Eur Heart J Acute Cardiovasc Care. 2019;8:208-217.
16. Fregni F. Sample Size Calculation. Clinical Thinking in Clinical Research:Applied Theory and Practice Using Case Studies. New York:Oxford University Press. 2018:225-242.
17. Gu J, Zhuo Y, Liu TJ, et al. Balloon Deflation Strategy during Primary Percutaneous Coronary Intervention in Acute ST-Segment Elevation Myocardial Infarction:A Randomized Controlled Clinical Trial and Numerical Simulation-Based Analysis. Cardiol Res Pract. 2020;2020:4826073.
18. Loubeyre C, Morice MC, Lefevre T, Piechaud JF, Louvard Y, Dumas P. A randomized comparison of direct stenting with conventional stent implantation in selected patients with acute myocardial infarction. J Am Coll Cardiol. 2002;39:15-21.
19. Lemesle G, Sudre A, Bouallal R, et al. Impact of thrombus aspiration use and direct stenting on final myocardial blush score in patients presenting with ST-elevation myocardial infarction. Cardiovasc Revasc Med. 2010;11:149-154.
20. Sardella G, Mancone M, Nguyen BL, et al. The effect of thrombectomy on myocardial blush in primary angioplasty:the Randomized Evaluation of Thrombus Aspiration by two thrombectomy devices in acute Myocardial Infarction (RETAMI) trial. Catheter Cardiovasc Interv. 2008;71:84-91.
21. G DEL, Bellandi F, Huber K, et al. Early glycoprotein IIb-IIIa inhibitors in primary angioplasty-abciximab long-term results (EGYPT-ALT) cooperation:individual patient's data meta-analysis. J Thromb Haemost. 2011;9:2361-2370.
22. Marra MP, Corbetti F, Cacciavillani L, et al. Relationship between myocardial blush grades, staining, and severe microvascular damage after primary percutaneous coronary intervention a study performed with contrast-enhanced magnetic resonance in a large consecutive series of patients. Am Heart J. 2010;159:1124-1132.
23. Bajaj NS, Singh A, Zhou W, et al. Coronary Microvascular Dysfunction, Left Ventricular Remodeling, and Clinical Outcomes in Patients With Chronic Kidney Impairment. Circulation. 2020;141:21-33.
24. De Luca G, van 't Hof AW, de Boer MJ, et al. Time-to-treatment significantly affects the extent of ST-segment resolution and myocardial blush in patients with acute myocardial infarction treated by primary angioplasty. Eur Heart J. 2004;25:1009-1013.
25. Ng VG, Lansky AJ, Toro S, et al. Prognostic utility of myocardial blush grade after PCI in patients with NSTE-ACS:Analysis from the ACUITY trial. Catheter Cardiovasc Interv. 2016;88:215-224.
26. Bottner RK, Morea CJ, Green CR, Renzi RH, Kent KM, Krucoff MW. Quantitation of ischemia during total coronary occlusion with computer-assisted high resolution ST-segment monitoring:effect of collateral flow. J Electrocardiol. 1987;20 Suppl:104-106.
27. Brodie BR, Stuckey TD, Hansen C, et al. Relation between electrocardiographic ST-segment resolution and early and late outcomes after primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol. 2005;95:343-348.
28. Verouden NJ, Haeck JD, Kuijt WJ, et al. Clinical and angiographic predictors of ST-segment recovery after primary percutaneous coronary intervention. Am J Cardiol. 2010;105:1692-1697.
29. Lefevre T, Garcia E, Reimers B, et al. X-sizer for thrombectomy in acute myocardial infarction improves ST-segment resolution:results of the X-sizer in AMI for negligible embolization and optimal ST resolution (X AMINE ST) trial. J Am Coll Cardiol. 2005;46:246-252.
30. De Luca G, Ernst N, van 't Hof AW, et al. Preprocedural Thrombolysis in Myocardial Infarction (TIMI) flow significantly affects the extent of ST-segment resolution and myocardial blush in patients with acute anterior myocardial infarction treated by primary angioplasty. Am Heart J. 2005;150:827-831.
31. Brener SJ, Dizon JM, Mehran R, et al. Complementary prognostic utility of myocardial blush grade and ST-segment resolution after primary percutaneous coronary intervention:analysis from the HORIZONS-AMI trial. Am Heart J. 2013;166:676-683.
* Corresponding author: Servicio de Cardiología, Hospital de Cabueñes, Avenida Los Prados 395, 33203 Gijón, Spain.
E-mail address: inigo.lozano@gmail.com (Í. Lozano).











