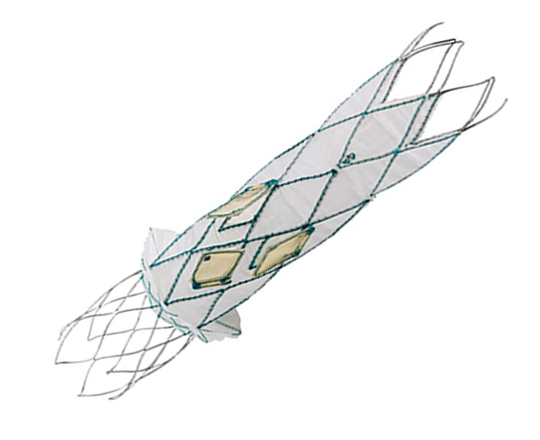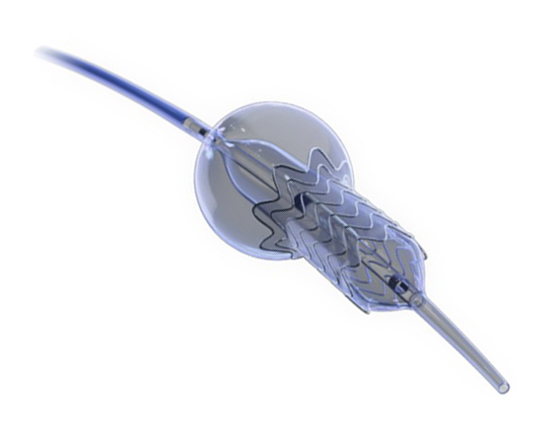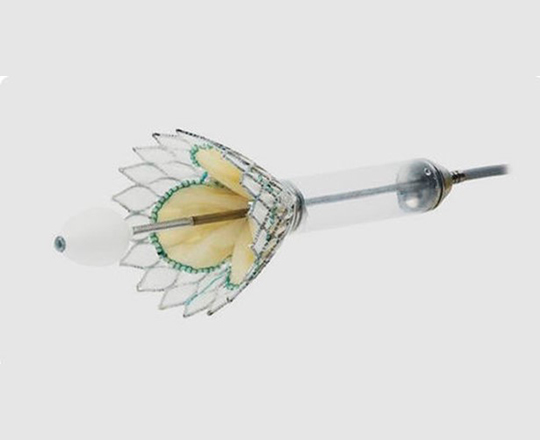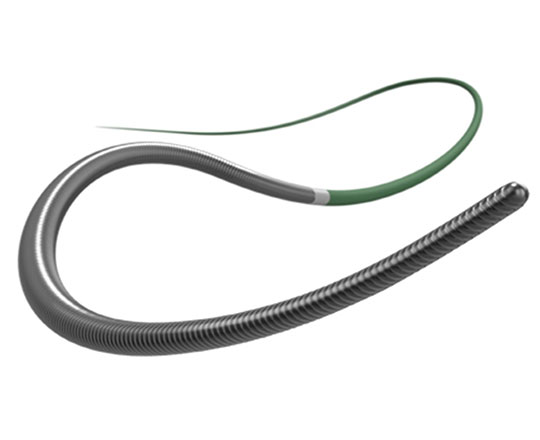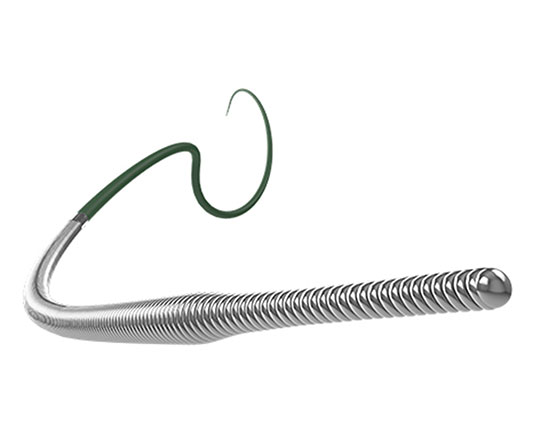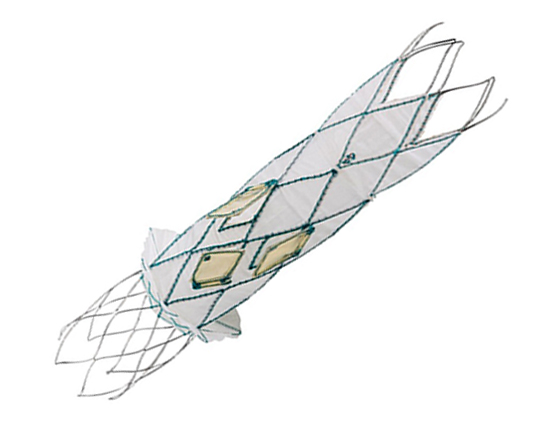
The Trillium device (Innoventric, Israel) is an endoprosthesis for tricuspid regurgitation that is positioned to run from the superior vena cava to the inferior vena cava. It has multiple covered fenestrations, which allow the inflow of blood from the venous system toward the right atrium through three windows, avoiding backflow to the venous system. It comes with a 24 Fr deployment system.
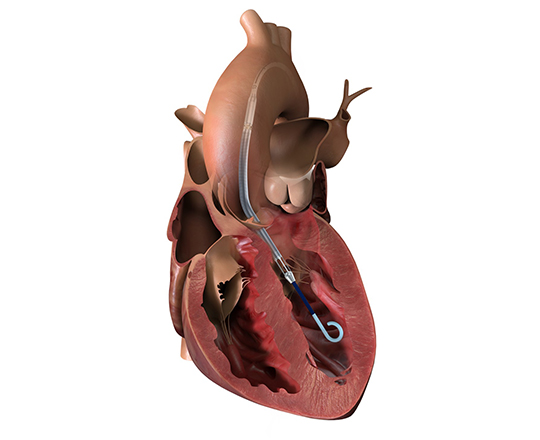
The prosthesis has fluoroscopic markers along its structure to facilitate its positioning. The procedure is strictly fluoroscopic and is performed via transfemoral venous access.
The cylindrical shape of the device facilitates its positioning it in the right atrium, as it can rotate without limiting the valves’ opening, providing greater safety to this novel technology. The Trillium valve system includes a distal skirt, designed to block inferior vena cava backflow without blocking inflow from the hepatic vein in patients with a very short distance between the hepatic veins and the right atrial cavity.
It can be positioned on top of a previously-implanted pacemaker and it can be used along with implantable cardioverter-defibrillator leads.
Currently, the Trillium system is undergoing its first-in-human clinical trial (NCT 04289870), with more than 10 patients treated in Germany, Belgium, and Israel to date. In early 2022, it began to be used in Spain and other EU countries as part of its clinical development to obtain the CE mark. There are very high expectations for this valve.
Keywords: Tricuspid regurgitation, percutaneous prosthetic valve.